Membrane Localization of Human Equilibrative Nucleoside Transporter 1 in Tumor Cells May Predict Response to Adjuvant Gemcitabine in Resected Cholangiocarcinoma Patients
- PMID: 27032872
- PMCID: PMC4861359
- DOI: 10.1634/theoncologist.2015-0356
Membrane Localization of Human Equilibrative Nucleoside Transporter 1 in Tumor Cells May Predict Response to Adjuvant Gemcitabine in Resected Cholangiocarcinoma Patients
Abstract
Background: The use of gemcitabine as an adjuvant modality for cholangiocarcinoma (CC) is increasing, but limited data are available on predictive biomarkers of response. Human equilibrative nucleoside transporter 1 (hENT-1) is the major transporter involved in gemcitabine intracellular uptake. This study investigated the putative predictive role of hENT-1 localization in tumor cells of CC patients undergoing treatment with adjuvant gemcitabine.
Methods: Seventy-one consecutive patients with resected CC receiving adjuvant gemcitabine at our center were retrospectively analyzed by immunohistochemistry for hENT-1 localization in tumor cells. The main outcome measure was disease-free survival (DFS). Hazard ratios (HRs) of relapse and associated 95% confidence intervals (CIs) were obtained from proportional hazards regression models stratified on quintiles of propensity score.
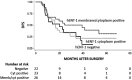
Results: Twenty-three (32.4%) cases were negative for hENT-1, 22 (31.0%) were positive in the cytoplasm only, and 26 (36.6%) showed concomitant cytoplasm/membrane staining. Patients with membrane hENT-1 had a longer DFS (HR 0.49, 95% CI 0.24-0.99, p = .046) than those who were negative or positive only in the cytoplasm of tumor cells. Notably, the association between DFS and membrane hENT-1 was dependent on the number of gemcitabine cycles (one to two cycles: HR 0.96, 95% CI 0.34-2.68; three to four cycles: HR 0.99, 95% CI 0.34-2.90; five to six cycles: HR 0.27, 95% CI 0.10-0.77).
Conclusion: hENT-1 localization on tumor cell membrane may predict response to adjuvant gemcitabine in CC patients receiving more than four cycles of chemotherapy. Further prospective randomized trials on larger populations are required to confirm these preliminary results, so that optimal gemcitabine-based chemotherapy may be tailored for CC patients in the adjuvant setting.
Implications for practice: Gemcitabine is becoming an increasingly used adjuvant modality in cholangiocarcinoma (CC), but limited data are available on predictive biomarkers of response. In this study, patients receiving more than four cycles of adjuvant gemcitabine and harboring Human equilibrative nucleoside transporter 1 (hENT-1, the major transporter involved in gemcitabine intracellular uptake) on tumor cell membrane had a longer disease-free survival compared with patients negative or positive for hENT-1 only in the cytoplasm of tumor cells. Overall these results may lay the basis for further prospective randomized trials based on a larger population of patients and may prove useful for tailoring appropriate gemcitabine-based chemotherapy for CC patients in the adjuvant setting.
Keywords: Adjuvant gemcitabine; Cholangiocarcinoma; Human equilibrative nucleoside transporter 1; Predictive biomarkers of response to chemotherapy.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures


Comment in
-
In Reply.Oncologist. 2016 Dec;21(12):e5-e6. doi: 10.1634/theoncologist.2016-0286. Epub 2016 Nov 2. Oncologist. 2016. PMID: 27807301 Free PMC article.
-
hENT-1 Expression and Localization Predict Outcome After Adjuvant Gemcitabine in Resected Cholangiocarcinoma Patients.Oncologist. 2016 Dec;21(12):e4. doi: 10.1634/theoncologist.2016-0262. Epub 2016 Nov 2. Oncologist. 2016. PMID: 27807304 Free PMC article.
References
-
- Anderson CD, Pinson CW, Berlin J, et al. Diagnosis and treatment of cholangiocarcinoma. The Oncologist. 2004;9:43–57. - PubMed
-
- Miyakawa S, Ishihara S, Horiguchi A, et al. Biliary tract cancer treatment: 5,584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg. 2009;16:1–7. - PubMed
-
- Murakami Y, Uemura K, Sudo T, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol. 2011;18:651–658. - PubMed
-
- Brandi G, Venturi M, Pantaleo MA, et al. Cholangiocarcinoma: Current opinion on clinical practice diagnostic and therapeutic algorithms: A review of the literature and a long-standing experience of a referral center. Dig Liver Dis. 2015;48:231–241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

