Src kinases play a novel dual role in acute pancreatitis affecting severity but no role in stimulated enzyme secretion
- PMID: 27033118
- PMCID: PMC4935475
- DOI: 10.1152/ajpgi.00349.2015
Src kinases play a novel dual role in acute pancreatitis affecting severity but no role in stimulated enzyme secretion
Abstract
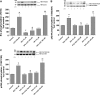
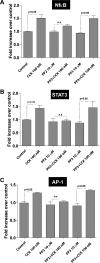
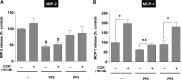
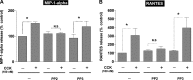
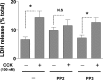
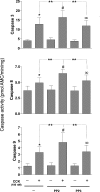
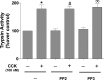
In pancreatic acinar cells, the Src family of kinases (SFK) is involved in the activation of several signaling cascades that are implicated in mediating cellular processes (growth, cytoskeletal changes, apoptosis). However, the role of SFKs in various physiological responses such as enzyme secretion or in pathophysiological processes such as acute pancreatitis is either controversial, unknown, or incompletely understood. To address this, in this study, we investigated the role/mechanisms of SFKs in acute pancreatitis and enzyme release. Enzyme secretion was studied in rat dispersed pancreatic acini, in vitro acute-pancreatitis-like changes induced by supramaximal COOH-terminal octapeptide of cholecystokinin (CCK). SFK involvement assessed using the chemical SFK inhibitor (PP2) with its inactive control, 4-amino-7-phenylpyrazol[3,4-d]pyrimidine (PP3), under experimental conditions, markedly inhibiting SFK activation. In CCK-stimulated pancreatic acinar cells, activation occurred of trypsinogen, various MAP kinases (p42/44, JNK), transcription factors (signal transducer and activator of transcription-3, nuclear factor-κB, activator protein-1), caspases (3, 8, and 9) inducing apoptosis, LDH release reflective of necrosis, and various chemokines secreted (monocyte chemotactic protein-1, macrophage inflammatory protein-1α, regulated on activation, normal T cell expressed and secreted). All were inhibited by PP2, not by PP3, except caspase activation leading to apoptosis, which was increased, and trypsin activation, which was unaffected, as was CCK-induced amylase release. These results demonstrate SFK activation is playing a dual role in acute pancreatitis, inhibiting apoptosis and promoting necrosis as well as chemokine/cytokine release inducing inflammation, leading to more severe disease, as well as not affecting secretion. Thus, our studies indicate that SFK is a key mediator of inflammation and pancreatic acinar cell death in acute pancreatitis, suggesting it could be a potential therapeutic target in acute pancreatitis.
Keywords: carboxy-terminal octapeptide of cholecystokinin; caspases; chemokines; pancreatic acini; signaling.
Copyright © 2016 the American Physiological Society.
Figures
Similar articles
-
Elucidation of the roles of the Src kinases in pancreatic acinar cell signaling.J Cell Biochem. 2015 Jan;116(1):22-36. doi: 10.1002/jcb.24895. J Cell Biochem. 2015. PMID: 25079913 Free PMC article.
-
Involvement of SRC family kinases in substance P-induced chemokine production in mouse pancreatic acinar cells and its significance in acute pancreatitis.J Pharmacol Exp Ther. 2009 May;329(2):418-28. doi: 10.1124/jpet.108.148684. Epub 2009 Feb 11. J Pharmacol Exp Ther. 2009. PMID: 19211920 Free PMC article.
-
PKCθ activation in pancreatic acinar cells by gastrointestinal hormones/neurotransmitters and growth factors is needed for stimulation of numerous important cellular signaling cascades.Biochim Biophys Acta. 2011 Dec;1813(12):2145-56. doi: 10.1016/j.bbamcr.2011.07.007. Epub 2011 Jul 23. Biochim Biophys Acta. 2011. PMID: 21810446 Free PMC article.
-
Pathogenic mechanisms of acute pancreatitis.Curr Opin Gastroenterol. 2012 Sep;28(5):507-15. doi: 10.1097/MOG.0b013e3283567f52. Curr Opin Gastroenterol. 2012. PMID: 22885948 Free PMC article. Review.
-
Apoptosis of pancreatic acinar cells in acute pancreatitis: is it good or bad?J Cell Mol Med. 2004 Jul-Sep;8(3):402-9. doi: 10.1111/j.1582-4934.2004.tb00330.x. J Cell Mol Med. 2004. PMID: 15491516 Free PMC article. Review.
Cited by
-
Targeting the complexity of Src signalling in the tumour microenvironment of pancreatic cancer: from mechanism to therapy.FEBS J. 2019 Sep;286(18):3510-3539. doi: 10.1111/febs.15011. Epub 2019 Aug 5. FEBS J. 2019. PMID: 31330086 Free PMC article. Review.
-
Pancreatic Cancer and Its Microenvironment-Recent Advances and Current Controversies.Int J Mol Sci. 2020 May 1;21(9):3218. doi: 10.3390/ijms21093218. Int J Mol Sci. 2020. PMID: 32370075 Free PMC article. Review.
-
Drug-induced pancreatitis: a real-world analysis of the FDA Adverse Event Reporting System and network pharmacology.Front Pharmacol. 2025 Apr 16;16:1564127. doi: 10.3389/fphar.2025.1564127. eCollection 2025. Front Pharmacol. 2025. PMID: 40308779 Free PMC article.
-
Ponatinib Protects Mice From Lethal Influenza Infection by Suppressing Cytokine Storm.Front Immunol. 2019 Jun 21;10:1393. doi: 10.3389/fimmu.2019.01393. eCollection 2019. Front Immunol. 2019. PMID: 31293574 Free PMC article.
-
Essential Role of Lyn in Fibrosis.Front Physiol. 2016 Aug 31;7:387. doi: 10.3389/fphys.2016.00387. eCollection 2016. Front Physiol. 2016. PMID: 27630579 Free PMC article.
References
-
- Beil M, Leser J, Lutz MP, Gukovskaya A, Seufferlein T, Lynch G, Pandol SJ, Adler G. Caspase 8-mediated cleavage of plectin precedes F-actin breakdown in acinar cells during pancreatitis. Am J Physiol Gastrointest Liver Physiol 282: G450–G460, 2002. - PubMed
-
- Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr Drug Targets Inflamm Allergy 1: 343–351, 2002. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

