New and emerging prognostic and predictive genetic biomarkers in B-cell precursor acute lymphoblastic leukemia
- PMID: 27033238
- PMCID: PMC5004393
- DOI: 10.3324/haematol.2015.141101
New and emerging prognostic and predictive genetic biomarkers in B-cell precursor acute lymphoblastic leukemia
Abstract
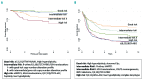
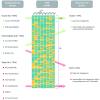
Acute lymphoblastic leukemia (ALL) is a heterogeneous disease at the genetic level. Chromosomal abnormalities are used as diagnostic, prognostic and predictive biomarkers to provide subtype, outcome and drug response information. t(12;21)/ETV6-RUNX1 and high hyper-diploidy are good-risk prognostic biomarkers whereas KMT2A(MLL) translocations, t(17;19)/TCF3-HLF, haploidy or low hypodiploidy are high-risk biomarkers. t(9;22)/BCR-ABL1 patients require targeted treatment (imatinib/dasatinib), whereas iAMP21 patients achieve better outcomes when treated intensively. High-risk genetic biomarkers are four times more prevalent in adults compared to children. The application of genomic technologies to cases without an established abnormality (B-other) reveals copy number alterations which can be used either individually or in combination as prognostic biomarkers. Transcriptome sequencing studies have identified a network of fusion genes involving kinase genes -ABL1,ABL2,PDGFRB,CSF1R,CRLF2,JAK2 and EPOR in-vitro and in-vivo studies along with emerging clinical observations indicate that patients with a kinase-activating aberration may respond to treatment with small molecular inhibitors like imatinib/dasatinib and ruxolitinib. Further work is required to determine the true frequency of these abnormalities across the age spectrum and the optimal way to incorporate such inhibitors into protocols. In conclusion, genetic biomarkers are playing an increasingly important role in the management of patients with ALL.
Copyright© Ferrata Storti Foundation.
Figures


References
-
- Vora A, Goulden N, Mitchell C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15(8):809–818. - PubMed
-
- Vora A, Goulden N, Wade R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14(3):199–209. - PubMed
-
- Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111(4):1827–1833. - PubMed
-
- Moorman AV, Chilton L, Wilkinson J, Ensor HM, Bown N, Proctor SJ. A population-based cytogenetic study of adults with acute lymphoblastic leukemia. Blood. 2010;115(2):206–214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

