Therapeutic hypothermia protects against ischemia-induced impairment of synaptic plasticity following juvenile cardiac arrest in sex-dependent manner
- PMID: 27033251
- PMCID: PMC5217704
- DOI: 10.1016/j.neuroscience.2016.03.052
Therapeutic hypothermia protects against ischemia-induced impairment of synaptic plasticity following juvenile cardiac arrest in sex-dependent manner
Abstract
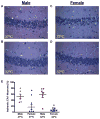
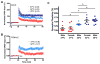
Pediatric cardiac arrest (CA) often leads to poor neurologic outcomes, including deficits in learning and memory. The only approved treatment for CA is therapeutic hypothermia, although its utility in the pediatric population remains unclear. This study analyzed the effect of mild therapeutic hypothermia after CA in juvenile mice on hippocampal neuronal injury and the cellular model of learning and memory, termed long-term potentiation (LTP). Juvenile mice were subjected to cardiac arrest and cardiopulmonary resuscitation (CA/CPR) followed by normothermia (37°C) and hypothermia (30°C, 32°C). Histological injury of hippocampal CA1 neurons was performed 3days after resuscitation using hematoxylin and eosin (H&E) staining. Field excitatory post-synaptic potentials (fEPSPs) were recorded from acute hippocampal slices 7days after CA/CPR to determine LTP. Synaptic function was impaired 7days after CA/CPR. Mice exposed to hypothermia showed equivalent neuroprotection, but exhibited sexually dimorphic protection against ischemia-induced impairment of LTP. Hypothermia (32°C) protects synaptic plasticity more effectively in females, with males requiring a deeper level of hypothermia (30°C) for equivalent protection. In conclusion, male and female juvenile mice exhibit equivalent neuronal injury following CA/CPR and hypothermia protects both males and females. We made the surprising finding that juvenile mice have a sexually dimorphic response to mild therapeutic hypothermia protection of synaptic function, where males may need a deeper level of hypothermia for equivalent synaptic protection.
Keywords: cardiac arrest; global ischemia; hypothermia; pediatric; synaptic function; synaptic plasticity.
Copyright © 2016 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

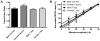
Similar articles
-
A novel mouse model of pediatric cardiac arrest and cardiopulmonary resuscitation reveals age-dependent neuronal sensitivities to ischemic injury.J Neurosci Methods. 2014 Jan 30;222:34-41. doi: 10.1016/j.jneumeth.2013.10.015. Epub 2013 Nov 2. J Neurosci Methods. 2014. PMID: 24192226 Free PMC article.
-
Cardiac Arrest Induces Ischemic Long-Term Potentiation of Hippocampal CA1 Neurons That Occludes Physiological Long-Term Potentiation.Neural Plast. 2018 Apr 26;2018:9275239. doi: 10.1155/2018/9275239. eCollection 2018. Neural Plast. 2018. PMID: 29853851 Free PMC article.
-
Endogenous Recovery of Hippocampal Function Following Global Cerebral Ischemia in Juvenile Female Mice Is Influenced by Neuroinflammation and Circulating Sex Hormones.Neural Plast. 2025 May 9;2025:6103242. doi: 10.1155/np/6103242. eCollection 2025. Neural Plast. 2025. PMID: 40386541 Free PMC article.
-
Resuscitative hypothermia.Crit Care Med. 1996 Feb;24(2 Suppl):S81-9. Crit Care Med. 1996. PMID: 8608709 Review.
-
Hypothermia after cardiac arrest.Prog Cardiovasc Dis. 2009 Sep-Oct;52(2):168-79. doi: 10.1016/j.pcad.2009.07.001. Prog Cardiovasc Dis. 2009. PMID: 19732608 Review.
Cited by
-
Interaction between gender and post resuscitation interventions on neurological outcome in an asphyxial rat model of cardiac arrest.BMC Cardiovasc Disord. 2021 Sep 16;21(1):441. doi: 10.1186/s12872-021-02262-5. BMC Cardiovasc Disord. 2021. PMID: 34530726 Free PMC article.
-
Sex Differences in Brain Injury and Repair in Newborn Infants: Clinical Evidence and Biological Mechanisms.Front Pediatr. 2019 Jun 26;7:211. doi: 10.3389/fped.2019.00211. eCollection 2019. Front Pediatr. 2019. PMID: 31294000 Free PMC article. Review.
-
Therapeutic Hypothermia Provides Variable Protection against Behavioral Deficits after Neonatal Hypoxia-Ischemia: A Potential Role for Brain-Derived Neurotrophic Factor.Dev Neurosci. 2017;39(1-4):257-272. doi: 10.1159/000454949. Epub 2017 Feb 15. Dev Neurosci. 2017. PMID: 28196356 Free PMC article.
-
Neonatal cardiopulmonary resuscitation: is ROSC enough?Pediatr Res. 2024 Oct;96(5):1123-1124. doi: 10.1038/s41390-024-03398-8. Epub 2024 Jul 24. Pediatr Res. 2024. PMID: 39048670 Free PMC article. No abstract available.
-
Juvenile cerebral ischemia reveals age-dependent BDNF-TrkB signaling changes: Novel mechanism of recovery and therapeutic intervention.J Cereb Blood Flow Metab. 2018 Dec;38(12):2223-2235. doi: 10.1177/0271678X18766421. Epub 2018 Apr 3. J Cereb Blood Flow Metab. 2018. PMID: 29611441 Free PMC article.
References
-
- Arrich J, Holzer M, Havel C, Mullner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. The Cochrane database of systematic reviews. 2012;9:CD004128. - PubMed
-
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
-
- Bliss TV, Collingridge GL, Laroche S. ZAP and ZIP, a story to forget. Science. 2006;313:1058–1059. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

