Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases
- PMID: 27033256
- PMCID: PMC4841706
- DOI: 10.1038/nrm.2016.27
Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases
Abstract
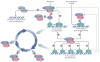
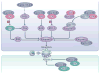
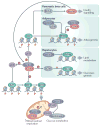
The roles of cyclins and their catalytic partners, the cyclin-dependent kinases (CDKs), as core components of the machinery that drives cell cycle progression are well established. Increasing evidence indicates that mammalian cyclins and CDKs also carry out important functions in other cellular processes, such as transcription, DNA damage repair, control of cell death, differentiation, the immune response and metabolism. Some of these non-canonical functions are performed by cyclins or CDKs, independently of their respective cell cycle partners, suggesting that there was a substantial divergence in the functions of these proteins during evolution.
Conflict of interest statement
The authors declare no competing interests.
Figures

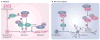
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

