Improved neurite outgrowth on central nervous system myelin substrate by siRNA-mediated knockdown of Nogo receptor
- PMID: 27033267
- PMCID: PMC4897850
- DOI: 10.1016/j.cjtee.2015.09.008
Improved neurite outgrowth on central nervous system myelin substrate by siRNA-mediated knockdown of Nogo receptor
Abstract
Purpose: To investigate the in vitro effect of short interfering RNAs (siRNAs) against Nogo receptor (NgR) on neurite outgrowth under an inhibitory substrate of central nervous system (CNS) myelin.
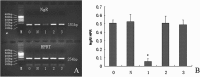
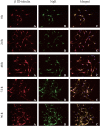
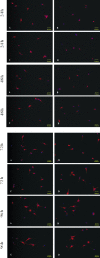
Methods: Three siRNA sequences against NgR were designed and transfected into cerebellar granule cells (CGCs) to screen for the most effcient sequence of NgR siRNA by using reverse transcription polymerase chain reaction (RT-PCR) and immunofluorescence staining. NgR siRNA sequence 1 was found the most efficient which was then transfected into the CGCs grown on CNS myelin substrate to observe its disinhibition for neurite outgrowth.
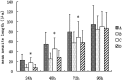
Results: Compared with the scrambled control sequence of siRNA, the NgR siRNA sequence 1 significantly decreased NgR mRNA level at 24 h and 48 h (p <0.05), which was recovered by 96 h after transfection. NgR immunoreactivity was also markedly reduced at 24 and 48 h after the transfection of siRNA sequence 1 compared with that before transfection (p<0.05). The NgR immunoreactivity was recovered after 72 h post-transfection. Moreover, the neurite outgrowth on the myelin substrate was greatly improved within 72 h after the transfection with siRNA sequence 1 compared with the scrambled sequence-transfected group or non-transfected group (p<0.05).
Conclusion: siRNA-mediated knockdown of NgR expression contributes to neurite outgrowth in vitro.
Figures




References
-
- Mukhopadhyay G., Doherty P., Walsh F.S. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. - PubMed
-
- Domeniconi M., Cao Z., Spencer T. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. - PubMed
-
- Borisoff J.F., Chan C.C., Hiebert G.W. Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci. 2003;22:405–416. - PubMed
-
- Berry M., Hunter A.S., Duncan A. Axon-glial relations during regeneration of axons in the adult rat anterior medullary velum. J Neurocytol. 1998;27:915–937. - PubMed
