Preclinical evaluation of RNAi as a treatment for transthyretin-mediated amyloidosis
- PMID: 27033334
- PMCID: PMC4898164
- DOI: 10.3109/13506129.2016.1160882
Preclinical evaluation of RNAi as a treatment for transthyretin-mediated amyloidosis
Abstract
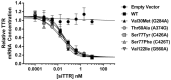
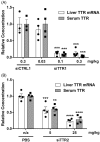
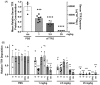
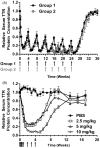
ATTR amyloidosis is a systemic, debilitating and fatal disease caused by transthyretin (TTR) amyloid accumulation. RNA interference (RNAi) is a clinically validated technology that may be a promising approach to the treatment of ATTR amyloidosis. The vast majority of TTR, the soluble precursor of TTR amyloid, is expressed and synthesized in the liver. RNAi technology enables robust hepatic gene silencing, the goal of which would be to reduce systemic levels of TTR and mitigate many of the clinical manifestations of ATTR that arise from hepatic TTR expression. To test this hypothesis, TTR-targeting siRNAs were evaluated in a murine model of hereditary ATTR amyloidosis. RNAi-mediated silencing of hepatic TTR expression inhibited TTR deposition and facilitated regression of existing TTR deposits in pathologically relevant tissues. Further, the extent of deposit regression correlated with the level of RNAi-mediated knockdown. In comparison to the TTR stabilizer, tafamidis, RNAi-mediated TTR knockdown led to greater regression of TTR deposits across a broader range of affected tissues. Together, the data presented herein support the therapeutic hypothesis behind TTR lowering and highlight the potential of RNAi in the treatment of patients afflicted with ATTR amyloidosis.
Keywords: GalNAc; gene silencing; lipid nanoparticle; siRNA; therapeutic.
Figures


Similar articles
-
Suppressing transthyretin production in mice, monkeys and humans using 2nd-Generation antisense oligonucleotides.Amyloid. 2016 Sep;23(3):148-157. doi: 10.1080/13506129.2016.1191458. Epub 2016 Jun 29. Amyloid. 2016. PMID: 27355239 Clinical Trial.
-
Liver-directed drugs for transthyretin-mediated amyloidosis.J Peripher Nerv Syst. 2022 Dec;27(4):228-237. doi: 10.1111/jns.12519. Epub 2022 Nov 16. J Peripher Nerv Syst. 2022. PMID: 36345805 Free PMC article. Review.
-
Leading RNA Interference Therapeutics Part 1: Silencing Hereditary Transthyretin Amyloidosis, with a Focus on Patisiran.Mol Diagn Ther. 2020 Feb;24(1):49-59. doi: 10.1007/s40291-019-00434-w. Mol Diagn Ther. 2020. PMID: 31701435 Review.
-
Efficiency of silencing RNA for removal of transthyretin V30M in a TTR leptomeningeal animal model.Amyloid. 2016 Dec;23(4):249-253. doi: 10.1080/13506129.2016.1256282. Epub 2016 Nov 24. Amyloid. 2016. PMID: 27884058
-
Advances in Studying the Pathologic Mechanisms and Treatment Strategies of Transthyretin Amyloidosis.J Cardiovasc Pharmacol. 2025 Mar 1;85(3):186-193. doi: 10.1097/FJC.0000000000001663. J Cardiovasc Pharmacol. 2025. PMID: 39739411 Review.
Cited by
-
GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics.Nucleic Acid Ther. 2018 Jun;28(3):109-118. doi: 10.1089/nat.2018.0736. Epub 2018 May 24. Nucleic Acid Ther. 2018. PMID: 29792572 Free PMC article. Review.
-
Advances in the Treatment of Cardiac Amyloidosis.Curr Treat Options Oncol. 2020 Apr 23;21(5):36. doi: 10.1007/s11864-020-00738-8. Curr Treat Options Oncol. 2020. PMID: 32328845 Free PMC article. Review.
-
A Novel Amino Lipid Series for mRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-human Primates.Mol Ther. 2018 Jun 6;26(6):1509-1519. doi: 10.1016/j.ymthe.2018.03.010. Epub 2018 Mar 14. Mol Ther. 2018. PMID: 29653760 Free PMC article.
-
Emerging Therapeutics for the Treatment of Light Chain and Transthyretin Amyloidosis.JACC Basic Transl Sci. 2019 Jun 24;4(3):438-448. doi: 10.1016/j.jacbts.2019.02.002. eCollection 2019 Jun. JACC Basic Transl Sci. 2019. PMID: 31312767 Free PMC article. Review.
-
A review of animal models utilized in preclinical studies of approved gene therapy products: trends and insights.Lab Anim Res. 2024 Apr 22;40(1):17. doi: 10.1186/s42826-024-00195-6. Lab Anim Res. 2024. PMID: 38649954 Free PMC article. Review.
References
-
- Blake CC, Swan ID, Rerat C, Berthou J, Laurent A, Rerat B. An X-ray study of the subunit structure of prealbumin. J Mol Biol. 1971;61:217–24. - PubMed
-
- Holmgren G, Steen L, Ekstedt J, Groth CG, Ericzon BG, Eriksson S, Andersen O, et al. Biochemical effect of liver transplantation in two Swedish patients with familial amyloidotic polyneuropathy (fap-met30). Clin Genet. 1991;40:242–6. - PubMed
-
- Kanda Y, Goodman DS, Canfield RE, Morgan FJ. The amino acid sequence of human plasma prealbumin. J Biol Chem. 1974;249:6796–805. - PubMed
-
- Nilsson SF, Rask L, Peterson PA. Studies on thyroid hormone-binding proteins. II. Binding of thyroid hormones, retinol-binding protein, and fluorescent probes to prealbumin and effects of thyroxine on prealbumin subunit self association. J Biol Chem. 1975;250:8554–63. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
