Indomethacin injury to the rat small intestine is dependent upon biliary secretion and is associated with overgrowth of enterococci
- PMID: 27033447
- PMCID: PMC4814884
- DOI: 10.14814/phy2.12725
Indomethacin injury to the rat small intestine is dependent upon biliary secretion and is associated with overgrowth of enterococci
Abstract
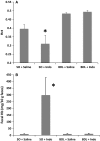
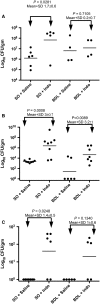
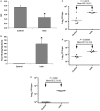
NSAIDuse is limited due to the drugs' toxicity to the gastrointestinal mucosa, an action incompletely understood. Lower gut injury induced byNSAIDs is dependent on bile secretion and is reported to increase the growth of a number of bacterial species, including an enterococcal species,Enterococcus faecalis This study examined the relationships between indomethacin (INDO)-induced intestinal injury/bleeding, small bowel overgrowth (SBO) and dissemination of enterococci, and the contribution of bile secretion to these pathological responses. Rats received either a sham operation (SO) or bile duct ligation (BDL) prior to administration of two daily subcutaneous doses of saline orINDO, and 24 h later, biopsies of ileum and liver were collected for plating on selective bacterial media. Fecal hemoglobin (Hb) and blood hematocrit (Hct) were measured to assess intestinal bleeding. Of the four treatment groups, onlySO/INDOrats experienced a significant 10- to 30-fold increase in fecal Hb and reduction in Hct, indicating thatBDLattenuatedINDO-induced intestinal injury/bleeding. Ileal enterococcal colony-forming units were significantly increased (500- to 1000-fold) inSO/INDOrats. Of all groups, only theSO/INDOrats demonstrated gut injury, and this was associated with enterococcal overgrowth of the gut and dissemination to the liver. We also demonstrated thatINDO-induced intestinal injury andE. faecalisovergrowth was independent of the route of administration of the drug, as similar findings were observed in rats orally dosed with theNSAID Bile secretion plays an important role inINDO-induced gut injury and appears to support enterococcal overgrowth of the intestine.NSAID-induced enterococcalSBOmay be involved either as a compensatory response to gut injury or with the pathogenic process itself and the subsequent development of sepsis.
Keywords: Bile acid; Enterococcus faecalis; non‐steroidal anti‐inflammatory drugs.
© 2016 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures

References
-
- Barrios, J. M. , and Lichtenberger L. M.. 2000. Role of biliary phosphatidylcholine in bile acid protection and NSAID injury of the ileal mucosa in rats. Gastroenterology 118:1179–1186. - PubMed
-
- Beck, W. S. , Schneider H. T., Dietzel K., Nuernberg B., and Brune K.. 1990. Gastrointestinal ulcerations induced by anti‐inflammatory drugs in rats. Physicochemical and biochemical factors involved. Arch. Toxicol. 64:210–217. - PubMed
-
- Blackler, R. W. , Gemici B., Manko A., and Wallace J. L.. 2014. NSAID‐gastroenteropathy: new aspects of pathogenesis and prevention. Curr. Opin. Pharmacol. 19C:11–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

