MLKL forms cation channels
- PMID: 27033670
- PMCID: PMC4856759
- DOI: 10.1038/cr.2016.26
MLKL forms cation channels
Abstract
The mixed lineage kinase domain-like (MLKL) protein is a key factor in tumor necrosis factor-induced necroptosis. Recent studies on necroptosis execution revealed a commitment role of MLKL in membrane disruption. However, our knowledge of how MLKL functions on membrane remains very limited. Here we demonstrate that MLKL forms cation channels that are permeable preferentially to Mg(2+) rather than Ca(2+) in the presence of Na(+) and K(+). Moreover, the N-terminal domain containing six helices (H1-H6) is sufficient to form channels. Using the substituted cysteine accessibility method, we further determine that helix H1, H2, H3, H5 and H6 are transmembrane segments, while H4 is located in the cytoplasm. Finally, MLKL-induced membrane depolarization and cell death exhibit a positive correlation to its channel activity. The Mg(2+)-preferred permeability and five transmembrane segment topology distinguish MLKL from previously identified Mg(2+)-permeable channels and thus establish MLKL as a novel class of cation channels.
Figures

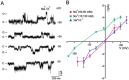
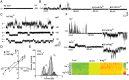
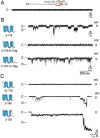

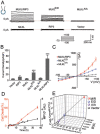
Comment in
-
Electrophysiologist shows a cation channel function of MLKL.Cell Res. 2016 Jun;26(6):643-4. doi: 10.1038/cr.2016.64. Epub 2016 May 27. Cell Res. 2016. PMID: 27229313 Free PMC article.
References
-
- Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature 2015; 517:311–320. - PubMed
-
- Sun L, Wang X. A new kind of cell suicide: mechanisms and functions of programmed necrosis. Trends Biochem Sci 2014; 39:587–593. - PubMed
-
- Grooten J, Goossens V. Vanhaesebroeck, et al. Cell membrane permeabilization and cellular collapse, followed by loss of dehydrogenase activity: early events in tumour necrosis factor-induced cytotoxicity. Cytokine 1993; 5:546–555. - PubMed
-
- Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol 1988; 141:2629–2634. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

