Molecular Basis of the Ligand Binding Specificity of αvβ8 Integrin
- PMID: 27033701
- PMCID: PMC4882426
- DOI: 10.1074/jbc.M116.719138
Molecular Basis of the Ligand Binding Specificity of αvβ8 Integrin
Abstract
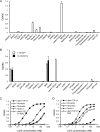
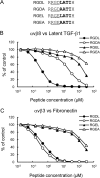
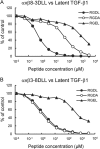
αvβ8 is an integrin that recognizes an Arg-Gly-Asp (RGD) motif and interacts with fibronectin, vitronectin, and latent TGF-β1. We comprehensively determined the binding activity of the αvβ8 integrin toward 25 secreted proteins having an RGD motif. The αvβ8 integrin strongly bound to latent TGF-β1 but showed marginal activity for other RGD-containing proteins, including fibronectin and vitronectin. Site-directed mutagenesis of latent TGF-β1 demonstrated that the high affinity binding of αvβ8 integrin to latent TGF-β1 was defined by Leu-218 immediately following the RGD motif within the latency-associated peptide of TGF-β1. Consistent with the critical role of Leu-218 in latent TGF-β1 recognition by αvβ8 integrin, a 9-mer synthetic peptide containing an RGDL sequence strongly inhibited interactions of latent TGF-β1 with αvβ8 integrin, whereas a 9-mer peptide with an RGDA sequence was ∼60-fold less inhibitory. Because αvβ3 integrin did not exhibit strong binding to latent TGF-β1 or distinguish between RGDL- and RGDA-containing peptides, we explored the mechanism by which the integrin β8 subunit defines the high affinity binding of latent TGF-β1 by αvβ8 integrin. Production of a series of swap mutants of integrin β8 and β3 subunits indicated that the high affinity binding of αvβ8 integrin with latent TGF-β1 was ensured by interactions between the Leu-218 residue and the β8 I-like domain, with the former serving as an auxiliary recognition residue defining the restricted ligand specificity of αvβ8 integrin toward latent TGF-β1. In support of this conclusion, high affinity binding toward the αvβ8 integrin was conferred on fibronectin by substitution of its RGDS motif with an RGDL sequence.
Keywords: RGD motif; cell adhesion; extracellular matrix; fibronectin; integrin; transforming growth factor beta (TGF-β).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






References
-
- Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 - PubMed
-
- Takagi J. (2007) Structural basis for ligand recognition by integrins. Curr. Opin. Cell Biol. 19, 557–564 - PubMed
-
- Brooks P. C., Clark R. A., and Cheresh D. A. (1994) Requirement of vascular integrin αvβ3 for angiogenesis. Science 264, 569–571 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

