Efficacy and toxicity of aerosolised colistin in ventilator-associated pneumonia: a prospective, randomised trial
- PMID: 27033711
- PMCID: PMC4816935
- DOI: 10.1186/s13613-016-0127-7
Efficacy and toxicity of aerosolised colistin in ventilator-associated pneumonia: a prospective, randomised trial
Abstract
Background: Cases of ventilator-associated pneumonia (VAP) due to multidrug-resistant (MDR) gram-negative bacilli (GNB) mainly Acinetobacter baumannii, Pseudomonas aeruginosa and enterobacteria are common in hospitalised patients of Tunisian intensive care units (ICUs). Parenteral colistin has been used for the therapy of VAP caused by MDR GNB at Tunisian hospitals over the past few years with a favourable clinical response. However, its use fell out of favour because of the reported drug-related nephrotoxicity and neurotoxicity.
Objectives: To determine whether aerosolised (AS) colistin was beneficial and safe in therapy of gram-negative VAP.
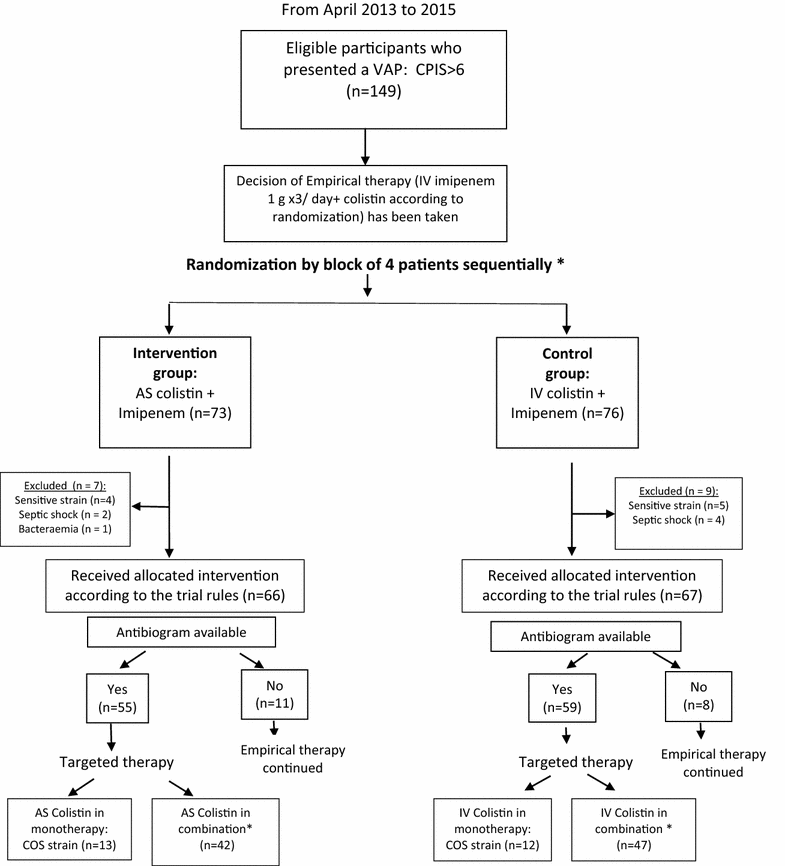
Methods: This was a randomised, single-blind study, in 149 critically ill adults who developed gram-negative VAP. Included patients were divided into two groups whether they received AS colistin (intervention group; n = 73) or intravenous (IV) colistin (control group; n = 76). AS colistin was given as 4 million units (MU) by nebulisation three times per 24 h. IV colistin was given as a loading dose of 9 MU followed by 4.5 MU two times per 24 h. Patients were followed during 28 days. Primary outcome was cure of VAP assessed at day 14 of therapy and defined as resolution of clinical signs of VAP and bacteriological eradication. Secondary outcomes were incidence of acute renal failure (ARF), mechanical ventilation length, ICU length of stay and 28-day mortality. Results were analysed based on intention-to-treat concept.
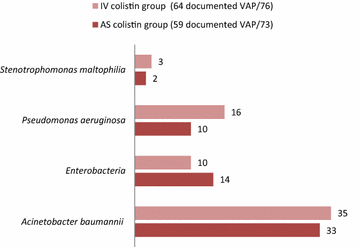
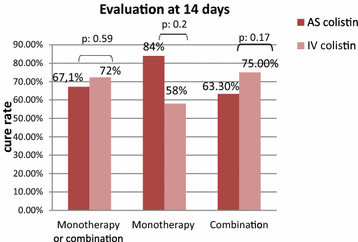
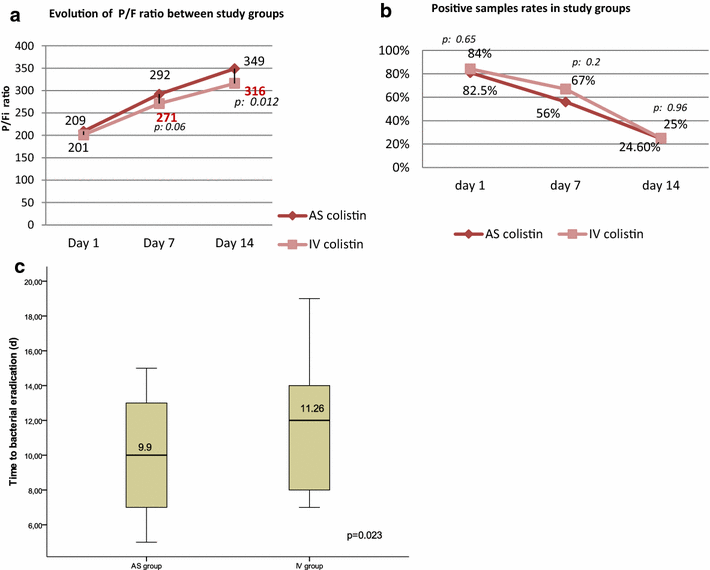
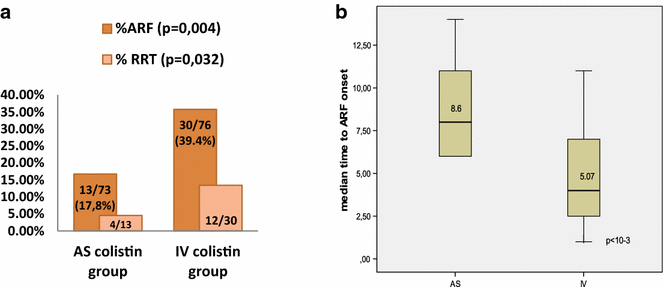
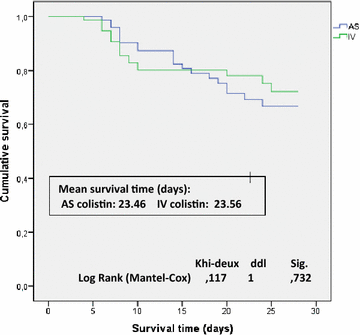
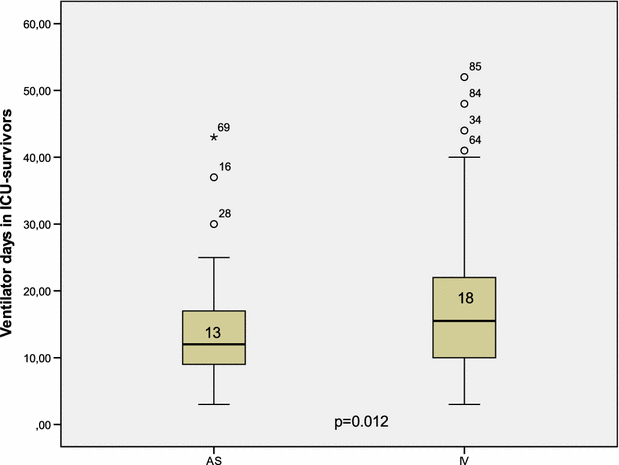
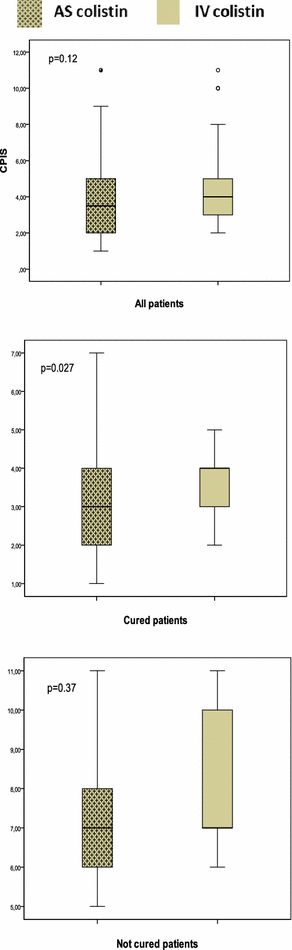
Results: The patient's baseline characteristics and distribution of pathogens VAP in both groups were similar. The clinical cure rate was 67.1 % in AS group and 72 % in IV group (p = 0.59). When administered in monotherapy or in combination, the AS regimen was as effective as IV regimen. Patients in AS group had significantly lower incidence of ARF (17.8 vs 39.4 %, p = 0.004), more favourable improvement of P/F ratio (349 vs 316 at day 14, p = 0.012), shortened time to bacterial eradication (TBE) (9.89 vs 11.26 days, p = 0.023) and earlier weaning from ventilator in ICU survivors with a mean gain in ventilator-free days of 5 days. No difference was shown in the length of stay and the 28-day mortality.
Conclusion: Aerosolised colistin seems to be beneficial. It provided a therapeutic effectiveness non-inferior to parenteral colistin in therapy of MDR bacilli VAP with a lower nephrotoxicity, a better improvement of P/F ratio, a shortened bacterial eradication time and earlier weaning from ventilator in ICU survivors. Trial registration ClinicalTrials.gov Identifier: NCT02683603.
Keywords: Aerosol; Colistin; Intravenous; Nephrotoxicity; Ventilator-associated pneumonia.
Figures
References
-
- Nguile-Makao M, Zahar JR, Français A. Attributable mortality of ventilator-associated pneumonia: respective impact of main characteristics at ICU admission and VAP onset using conditional logistic regression and multi-state models. Intensive Care Med. 2010;36:781–789. doi: 10.1007/s00134-010-1824-6. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

