Generation of cloned mice and nuclear transfer embryonic stem cell lines from urine-derived cells
- PMID: 27033801
- PMCID: PMC4817122
- DOI: 10.1038/srep23808
Generation of cloned mice and nuclear transfer embryonic stem cell lines from urine-derived cells
Abstract
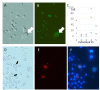
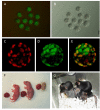
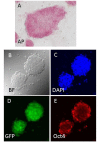
Cloning animals by nuclear transfer provides the opportunity to preserve endangered mammalian species. However, there are risks associated with the collection of donor cells from the body such as accidental injury to or death of the animal. Here, we report the production of cloned mice from urine-derived cells collected noninvasively. Most of the urine-derived cells survived and were available as donors for nuclear transfer without any pretreatment. After nuclear transfer, 38-77% of the reconstructed embryos developed to the morula/blastocyst, in which the cell numbers in the inner cell mass and trophectoderm were similar to those of controls. Male and female cloned mice were delivered from cloned embryos transferred to recipient females, and these cloned animals grew to adulthood and delivered pups naturally when mated with each other. The results suggest that these cloned mice had normal fertility. In additional experiments, 26 nuclear transfer embryonic stem cell lines were established from 108 cloned blastocysts derived from four mouse strains including inbreds and F1 hybrids with relatively high success rates. Thus, cells derived from urine, which can be collected noninvasively, may be used in the rescue of endangered mammalian species by using nuclear transfer without causing injury to the animal.
Figures

References
-
- Cibelli J. B. et al.. Principles of Cloning. Second edn, (Academic Press, 2014).
-
- Yang X. et al.. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat Genet 39, 295–302 (2007). - PubMed
-
- Loi P. et al.. Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nat Biotechnol 19, 962–964 (2001). - PubMed
-
- Lanza R. P. et al.. Cloning of an endangered species (Bos gaurus) using interspecies nuclear transfer. Cloning 2, 79–90 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

