Establishment of prostate cancer spheres from a prostate cancer cell line after phenethyl isothiocyanate treatment and discovery of androgen-dependent reversible differentiation between sphere and neuroendocrine cells
- PMID: 27034170
- PMCID: PMC5041999
- DOI: 10.18632/oncotarget.8440
Establishment of prostate cancer spheres from a prostate cancer cell line after phenethyl isothiocyanate treatment and discovery of androgen-dependent reversible differentiation between sphere and neuroendocrine cells
Abstract
Prostate cancer can transform from androgen-responsive to an androgen-independent phenotype. The mechanism responsible for the transformation remains unclear. We studied the effects of an epigenetic modulator, phenethyl isothiocyanate (PEITC), on the androgen-responsive LNCaP cells. After treatment with PEITC, floating spheres were formed with characteristics of prostate cancer stem cells (PCSC). These spheres were capable of self-renewal in media with and without androgen. They have been maintained in both types of media as long term cultures. Upon androgen deprivation, the adherent spheres differentiated to neuroendocrine cells (NEC) with decreased proliferation, expression of androgen receptor, and PSA. NEC reverse differentiated to spheres when androgen was replenished. The sphere cells expressed surface marker CD44 and had enhanced histone H3K4 acetylation, DNMT1 down-regulation and GSTP1 activation. We hypothesize that PEITC-mediated alteration in epigenomics of LNCaP cells may give rise to sphere cells, whereas reversible androgenomic alterations govern the shuttling between sphere PCSC and progeny NEC. Our findings identify unrecognized properties of prostate cancer sphere cells with multi-potential plasticity. This system will facilitate development of novel therapeutic agents and allow further exploration into epigenomics and androgenomics governing the transformation to hormone refractory prostate cancer.
Keywords: androgen; epigenome; prostate cancer; sphere; stem cells.
Conflict of interest statement
The authors declare no conflicts.
Figures
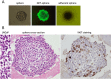
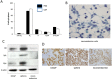




Similar articles
-
Repression of androgen receptor in prostate cancer cells by phenethyl isothiocyanate.Carcinogenesis. 2006 Oct;27(10):2124-32. doi: 10.1093/carcin/bgl075. Epub 2006 May 16. Carcinogenesis. 2006. PMID: 16704988
-
Enrichment of putative prostate cancer stem cells after androgen deprivation: upregulation of pluripotency transactivators concurs with resistance to androgen deprivation in LNCaP cell lines.Prostate. 2013 Sep;73(13):1378-90. doi: 10.1002/pros.22685. Epub 2013 May 31. Prostate. 2013. PMID: 23728788
-
Dual action on promoter demethylation and chromatin by an isothiocyanate restored GSTP1 silenced in prostate cancer.Mol Carcinog. 2007 Jan;46(1):24-31. doi: 10.1002/mc.20258. Mol Carcinog. 2007. PMID: 16921492
-
Prostate cancer chemopreventive activity of phenethyl isothiocyanate through epigenetic regulation (review).Int J Oncol. 2010 Sep;37(3):533-9. doi: 10.3892/ijo_00000702. Int J Oncol. 2010. PMID: 20664922 Review.
-
Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model.Prostate. 1996 Feb;28(2):98-106. doi: 10.1002/(SICI)1097-0045(199602)28:2<98::AID-PROS4>3.0.CO;2-J. Prostate. 1996. PMID: 8604398 Review.
Cited by
-
Involvement of breast cancer stem cells in tumor angiogenesis.Oncol Lett. 2017 Dec;14(6):8150-8155. doi: 10.3892/ol.2017.7238. Epub 2017 Oct 20. Oncol Lett. 2017. PMID: 29344258 Free PMC article.
-
Phenethyl Isothiocyanate, a Dual Activator of Transcription Factors NRF2 and HSF1.Mol Nutr Food Res. 2018 Sep;62(18):e1700908. doi: 10.1002/mnfr.201700908. Epub 2018 Jun 19. Mol Nutr Food Res. 2018. PMID: 29710398 Free PMC article. Review.
-
Preclinical Models of Neuroendocrine Neoplasia.Cancers (Basel). 2022 Nov 17;14(22):5646. doi: 10.3390/cancers14225646. Cancers (Basel). 2022. PMID: 36428741 Free PMC article. Review.
-
Development of human prostate cancer stem cells involves epigenomic alteration and PI3K/AKT pathway activation.Exp Hematol Oncol. 2020 Jun 11;9:12. doi: 10.1186/s40164-020-00168-0. eCollection 2020. Exp Hematol Oncol. 2020. PMID: 32537260 Free PMC article.
References
-
- Santos AF, Huang H, Tindall DJ. The androgen receptor: a potential target for therapy of prostate cancer. Steroids. 2004;69:79–85. - PubMed
-
- Taplin ME, Balk SP. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem. 2004;91:483–490. - PubMed
-
- Edwards J, Bartlett JM. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 2: Androgen-receptor cofactors and bypass pathways. BJU international. 2005;95:1327–1335. - PubMed
-
- Kinoshita H, Shi Y, Sandefur C, Meisner LF, Chang C, Choon A, Reznikoff CR, Bova GS, Friedl A, Jarrard DF. Methylation of the androgen receptor minimal promoter silences transcription in human prostate cancer. Cancer Res. 2000;60:3623–3630. - PubMed
-
- Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, Angelopoulou R, Rosen JM, Greenberg NM. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–4102. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

