The TAM family receptor tyrosine kinase TYRO3 is a negative regulator of type 2 immunity
- PMID: 27034374
- PMCID: PMC4935984
- DOI: 10.1126/science.aaf1358
The TAM family receptor tyrosine kinase TYRO3 is a negative regulator of type 2 immunity
Abstract
Host responses against metazoan parasites or an array of environmental substances elicit type 2 immunity. Despite its protective function, type 2 immunity also drives allergic diseases. The mechanisms that regulate the magnitude of the type 2 response remain largely unknown. Here, we show that genetic ablation of a receptor tyrosine kinase encoded byTyro3in mice or the functional neutralization of its ortholog in human dendritic cells resulted in enhanced type 2 immunity. Furthermore, the TYRO3 agonist PROS1 was induced in T cells by the quintessential type 2 cytokine, interleukin-4. T cell-specificPros1knockouts phenocopied the loss ofTyro3 Thus, a PROS1-mediated feedback from adaptive immunity engages a rheostat, TYRO3, on innate immune cells to limit the intensity of type 2 responses.
Copyright © 2016, American Association for the Advancement of Science.
Figures

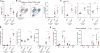
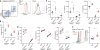

References
-
- Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. Cell. 2007;131:1124–1136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K23 HL004464/HL/NHLBI NIH HHS/United States
- HL104608/HL/NHLBI NIH HHS/United States
- HL117004/HL/NHLBI NIH HHS/United States
- HL078885/HL/NHLBI NIH HHS/United States
- HL004464/HL/NHLBI NIH HHS/United States
- P30 CA016359/CA/NCI NIH HHS/United States
- R01 HL088133/HL/NHLBI NIH HHS/United States
- HL088133/HL/NHLBI NIH HHS/United States
- R37 AR040072/AR/NIAMS NIH HHS/United States
- T32 AI007019/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- MD006902/MD/NIMHD NIH HHS/United States
- R01 AI095289/AI/NIAID NIH HHS/United States
- R01 HL078885/HL/NHLBI NIH HHS/United States
- R01 GM083204/GM/NIGMS NIH HHS/United States
- R01 HL104608/HL/NHLBI NIH HHS/United States
- R01 AI089824/AI/NIAID NIH HHS/United States
- T32 GM007205/GM/NIGMS NIH HHS/United States
- R01 HL117004/HL/NHLBI NIH HHS/United States
- P60 MD006902/MD/NIMHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

