Murine Models of Epstein-Barr Virus-Associated Lymphomagenesis
- PMID: 27034395
- PMCID: PMC6302257
- DOI: 10.1093/ilar/ilv074
Murine Models of Epstein-Barr Virus-Associated Lymphomagenesis
Abstract
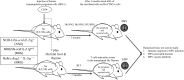
The Epstein-Barr virus (EBV) is a B-lymphotropic gamma herpes virus associated with a number of malignancies. Most EBV-related cancers present complex medical management challenges; thus it has been essential to develop preclinical in vivo models allowing for the study of pathogenesis, prevention, and treatment of these diseases. Early in vivo models used nonhuman primates; however, such models were limited by the inability of EBV to achieve viral latency, availability, and cost. Immunodeficient mouse strains emerged as efficient models that allow for engraftment of human mononuclear cells and controlled evaluation of EBV-driven lymphoproliferative disease (EBV-LPD). By using highly immunodeficient strains of mice such as severe combined immune deficiency (SCID) and NOD/LtSz-scid ILrg(-/-)(NOG) mice, investigators have developed efficient platforms for evaluating pathogenesis of benign (HLH) and malignant (EBV-LPD) diseases associated with EBV. Humanized murine chimeric models have been essential tools for evaluating preventive strategies with vaccine and adoptive cellular approaches, as well as development of experimental therapeutic strategies. Manipulation of the human immune cells before engraftment or mutation of viral lytic and latent genes has enhanced our understanding of the oncogenic nature of EBV and the complexity of human immune responses to EBV. In this review, we discuss how the EBV murine models have evolved to become essential tools for studying the virology of EBV as it relates to human EBV-LPD pathogenesis, the immunobiology of innate and adaptive responses, and limitations of these models.
Keywords: Epstein-Barr virus; hu-PBL-SCID; humanized mice; mouse models; review.
© The Author 2016. Published by Oxford University Press on behalf of the Institute for Laboratory Animal Research. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Andreone P, Gramenzi A, Lorenzini S, Biselli M, Cursaro C, Pileri S, Bernardi M. 2003. Posttransplantation lymphoproliferative disorders. Arch Intern Med 163:1997–2004. - PubMed
-
- Baiocchi RA, Ross ME, Tan JC, Chou CC, Sullivan L, Haldar S, Monne M, Seiden MV, Narula SK, Sklar J. 1995. Lymphomagenesis in the SCID-hu mouse involves abundant production of human interleukin-10. Blood 85:1063–1074. - PubMed
-
- Baiocchi RA, Ward JS, Carrodeguas L, Eisenbeis CF, Peng R, Roychowdhury S, Vourganti S, Sekula T, O'Brien M, Moeschberger M, Caligiuri MA. 2001. GM-CSF and IL-2 induce specific cellular immunity and provide protection against Epstein-Barr virus lymphoproliferative disorder. J Clin Invest 108:887–894. - PMC - PubMed
-
- Bosma GC, Custer RP, Bosma MJ. 1983. A severe combined immunodeficiency mutation in the mouse. Nature 301:527–530. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical