JAK/STAT regulation of Aspergillus fumigatus corneal infections and IL-6/23-stimulated neutrophil, IL-17, elastase, and MMP9 activity
- PMID: 27034404
- PMCID: PMC4946614
- DOI: 10.1189/jlb.4A1015-483R
JAK/STAT regulation of Aspergillus fumigatus corneal infections and IL-6/23-stimulated neutrophil, IL-17, elastase, and MMP9 activity
Abstract
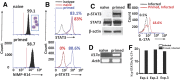
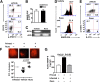
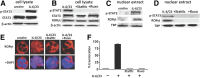
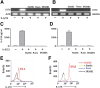
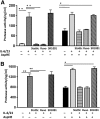
IL-6 and IL-23 (IL-6/23) induce IL-17A (IL-17) production by a subpopulation of murine and human neutrophils, resulting in autocrine IL-17 activation, enhanced production of reactive oxygen species, and increased fungal killing. As IL-6 and IL-23 receptors trigger JAK1, -3/STAT3 and JAK2/STAT3 phosphorylation, respectively, we examined the role of this pathway in a murine model of fungal keratitis and also examined neutrophil elastase and gelatinase (matrix metalloproteinase 9) activity by IL-6/23-stimulated human neutrophils in vitro. We found that STAT3 phosphorylation of neutrophils in Aspergillus fumigatus-infected corne as was inhibited by the JAK/STAT inhibitor Ruxolitinib, resulting in impaired fungal killing and decreased matrix metalloproteinase 9 activity. In vitro, we showed that fungal killing by IL-6/23-stimulated human peripheral blood neutrophils was impaired by JAK/STAT inhibitors Ruxolitinib and Stattic, and by the retinoic acid receptor-related orphan receptor γt inhibitor SR1001. This was also associated with decreased reactive oxygen species, IL-17A production, and retinoic acid receptor-related orphan receptor γt translocation to the nucleus. We also demonstrate that IL-6/23-activated neutrophils exhibit increased elastase and gelatinase (matrix metalloproteinase 9) activity, which is inhibited by Ruxolitinib and Stattic but not by SR1001. Taken together, these observations indicate that the regulation of activity of IL-17-producing neutrophils by JAK/STAT inhibitors impairs reactive oxygen species production and fungal killing activity but also blocks elastase and gelatinase activity that can cause tissue damage.
Keywords: RORγt; Ruxolitinb; SR1001; Stattic; keratitis.
© Society for Leukocyte Biology.
Figures


Similar articles
-
TSLP Produced by Aspergillus fumigatus-Stimulated DCs Promotes a Th17 Response Through the JAK/STAT Signaling Pathway in Fungal Keratitis.Invest Ophthalmol Vis Sci. 2020 Dec 1;61(14):24. doi: 10.1167/iovs.61.14.24. Invest Ophthalmol Vis Sci. 2020. PMID: 33346778 Free PMC article.
-
Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2.Nat Immunol. 2014 Feb;15(2):143-51. doi: 10.1038/ni.2797. Epub 2013 Dec 22. Nat Immunol. 2014. PMID: 24362892 Free PMC article.
-
GSDMD, an executor of pyroptosis, is involved in IL-1β secretion in Aspergillus fumigatus keratitis.Exp Eye Res. 2021 Jan;202:108375. doi: 10.1016/j.exer.2020.108375. Epub 2020 Dec 3. Exp Eye Res. 2021. PMID: 33279525
-
Pathogenic Aspergillus and Fusarium as important causes of blinding corneal infections - the role of neutrophils in fungal killing, tissue damage and cytokine production.Curr Opin Microbiol. 2021 Oct;63:195-203. doi: 10.1016/j.mib.2021.07.018. Epub 2021 Aug 19. Curr Opin Microbiol. 2021. PMID: 34419783 Free PMC article. Review.
-
The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances.Cell Cycle. 2010 May;9(9):1732-7. doi: 10.4161/cc.9.9.11297. Epub 2010 May 25. Cell Cycle. 2010. PMID: 20404546 Review.
Cited by
-
Luteolin Partially Inhibits LFA-1 Expression in Neutrophils Through the ERK Pathway.Inflammation. 2019 Feb;42(1):365-374. doi: 10.1007/s10753-018-0900-x. Inflammation. 2019. PMID: 30255285
-
Innate Lung Defense during Invasive Aspergillosis: New Mechanisms.J Innate Immun. 2017;9(3):271-280. doi: 10.1159/000455125. Epub 2017 Feb 24. J Innate Immun. 2017. PMID: 28231567 Free PMC article. Review.
-
EGF stimulates glioblastoma metastasis by induction of matrix metalloproteinase-9 in an EGFR-dependent mechanism.Oncotarget. 2017 Jul 27;8(39):65969-65982. doi: 10.18632/oncotarget.19622. eCollection 2017 Sep 12. Oncotarget. 2017. PMID: 29029486 Free PMC article.
-
ATF4 Involvement in TLR4 and LOX-1-Induced Host Inflammatory Response to Aspergillus fumigatus Keratitis.J Ophthalmol. 2018 Dec 13;2018:5830202. doi: 10.1155/2018/5830202. eCollection 2018. J Ophthalmol. 2018. PMID: 30647960 Free PMC article.
-
Integrating genetic and immune factors to uncover pathogenetic mechanisms of viral-associated pulmonary aspergillosis.mBio. 2024 Jun 12;15(6):e0198223. doi: 10.1128/mbio.01982-23. Epub 2024 Apr 23. mBio. 2024. PMID: 38651925 Free PMC article. Review.
References
-
- Wang L., Sun S., Jing Y., Han L., Zhang H., Yue J. (2009) Spectrum of fungal keratitis in central China. Clin. Experiment. Ophthalmol. 37, 763–771. - PubMed
-
- Xie L., Zhong W., Shi W., Sun S. (2006) Spectrum of fungal keratitis in north China. Ophthalmology 113, 1943–1948. - PubMed
-
- Fusarium Keratitis Investigation Team (2006) Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA 296, 953–963. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

