Functional and Transcriptional Characterization of Histone Deacetylase Inhibitor-Mediated Cardiac Adverse Effects in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes
- PMID: 27034410
- PMCID: PMC4835253
- DOI: 10.5966/sctm.2015-0279
Functional and Transcriptional Characterization of Histone Deacetylase Inhibitor-Mediated Cardiac Adverse Effects in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes
Abstract
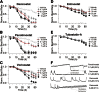
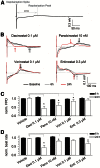
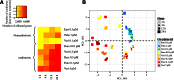
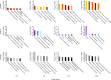
Histone deacetylase (HDAC) inhibitors possess therapeutic potential to reverse aberrant epigenetic changes associated with cancers, neurological diseases, and immune disorders. Unfortunately, clinical studies with some HDAC inhibitors displayed delayed cardiac adverse effects, such as atrial fibrillation and ventricular tachycardia. However, the underlying molecular mechanism(s) of HDAC inhibitor-mediated cardiotoxicity remains poorly understood and is difficult to detect in the early stages of preclinical drug development because of a delayed onset of effects. In the present study, we show for the first time in human induced pluripotent stem cell-derived cardiomyocytes (hiPS-CMs) that HDAC inhibitors (dacinostat, panobinostat, vorinostat, entinostat, and tubastatin-a) induce delayed dose-related cardiac dysfunction at therapeutic concentrations associated with cardiac adverse effects in humans. HDAC inhibitor-mediated delayed effects on the beating properties of hiPS-CMs developed after 12 hours by decreasing the beat rate, shortening the field potential duration, and inducing arrhythmic behavior under form of sustained contractions and fibrillation-like patterns. Transcriptional changes that are common between the cardiotoxic HDAC inhibitors but different from noncardiotoxic treatments identified cardiac-specific genes and pathways related to structural and functional changes in cardiomyocytes. Combining the functional data with epigenetic changes in hiPS-CMs allowed us to identify molecular targets that might explain HDAC inhibitor-mediated cardiac adverse effects in humans. Therefore, hiPS-CMs represent a valuable translational model to assess HDAC inhibitor-mediated cardiotoxicity and support identification of better HDAC inhibitors with an improved benefit-risk profile.
Significance: Histone deacetylase (HDAC) inhibitors are a promising class of drugs to treat certain cancers, autoimmune, and neurodegenerative diseases. However, treated patients can experience various cardiac adverse events such as hearth rhythm disorders. This study found that human induced pluripotent stem cell-derived cardiomyocytes (hiPS-CMs) can predict cardiac adverse events in patients caused by HDAC inhibitors. Furthermore, transcriptional changes at the level of gene expression supported the effects on the beating properties of hiPS-CMs and highlight targets that might cause these cardiac adverse effects. hiPS-CMs represent a valuable translational model to assess HDAC inhibitor-mediated cardiotoxicity and to support development of safer HDAC inhibitors.
Keywords: Arrhythmia; Cardiotoxicity; Histone deacetylase inhibitor; Human-induced stem cell-derived cardiomyocytes; Impedance.
©AlphaMed Press.
Figures

Similar articles
-
High Throughput Measurement of Ca++ Dynamics in Human Stem Cell-Derived Cardiomyocytes by Kinetic Image Cytometery: A Cardiac Risk Assessment Characterization Using a Large Panel of Cardioactive and Inactive Compounds.Toxicol Sci. 2015 Dec;148(2):503-16. doi: 10.1093/toxsci/kfv201. Epub 2015 Sep 9. Toxicol Sci. 2015. PMID: 26358003
-
Application of optical action potentials in human induced pluripotent stem cells-derived cardiomyocytes to predict drug-induced cardiac arrhythmias.J Pharmacol Toxicol Methods. 2017 Sep;87:53-67. doi: 10.1016/j.vascn.2017.05.001. Epub 2017 May 10. J Pharmacol Toxicol Methods. 2017. PMID: 28501647
-
Moving beyond the comprehensive in vitro proarrhythmia assay: Use of human-induced pluripotent stem cell-derived cardiomyocytes to assess contractile effects associated with drug-induced structural cardiotoxicity.J Appl Toxicol. 2018 Sep;38(9):1166-1176. doi: 10.1002/jat.3611. Epub 2018 Feb 27. J Appl Toxicol. 2018. PMID: 29484688 Review.
-
CSAHi study: Validation of multi-electrode array systems (MEA60/2100) for prediction of drug-induced proarrhythmia using human iPS cell-derived cardiomyocytes -assessment of inter-facility and cells lot-to-lot-variability.Regul Toxicol Pharmacol. 2016 Jun;77:75-86. doi: 10.1016/j.yrtph.2016.02.007. Epub 2016 Feb 13. Regul Toxicol Pharmacol. 2016. PMID: 26884090
-
Comprehensive in vitro cardiac safety assessment using human stem cell technology: Overview of CSAHi HEART initiative.J Pharmacol Toxicol Methods. 2017 Jan-Feb;83:42-54. doi: 10.1016/j.vascn.2016.09.004. Epub 2016 Sep 17. J Pharmacol Toxicol Methods. 2017. PMID: 27646297 Review.
Cited by
-
Single-cell profiling-guided combination therapy of c-Fos and histone deacetylase inhibitors in diffuse large B-cell lymphoma.Clin Transl Med. 2022 May;12(5):e798. doi: 10.1002/ctm2.798. Clin Transl Med. 2022. PMID: 35522945 Free PMC article.
-
Human iPSC-Cardiomyocytes as an Experimental Model to Study Epigenetic Modifiers of Electrophysiology.Cells. 2022 Jan 7;11(2):200. doi: 10.3390/cells11020200. Cells. 2022. PMID: 35053315 Free PMC article. Review.
-
Pharmacogenomics in drug-induced cardiotoxicity: Current status and the future.Front Cardiovasc Med. 2022 Oct 13;9:966261. doi: 10.3389/fcvm.2022.966261. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36312261 Free PMC article. Review.
-
Is Human-induced Pluripotent Stem Cell the Best Optimal?Chin Med J (Engl). 2018 Apr 5;131(7):852-856. doi: 10.4103/0366-6999.228231. Chin Med J (Engl). 2018. PMID: 29578130 Free PMC article. Review.
-
Chromatin dynamics underlying latent responses to xenobiotics.Toxicol Res (Camb). 2018 Feb 28;7(4):606-617. doi: 10.1039/c7tx00317j. eCollection 2018 Jul 1. Toxicol Res (Camb). 2018. PMID: 30090610 Free PMC article. Review.
References
-
- Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. - PubMed
-
- Spange S, Wagner T, Heinzel T, et al. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int J Biochem Cell Biol. 2009;41:185–198. - PubMed
-
- Choudhary C, Kumar C, Gnad F, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. - PubMed
-
- Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: Functional implications of phylogenetic analysis. J Mol Biol. 2004;338:17–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

