Modeling Cystic Fibrosis Using Pluripotent Stem Cell-Derived Human Pancreatic Ductal Epithelial Cells
- PMID: 27034411
- PMCID: PMC4835252
- DOI: 10.5966/sctm.2015-0276
Modeling Cystic Fibrosis Using Pluripotent Stem Cell-Derived Human Pancreatic Ductal Epithelial Cells
Abstract
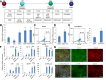
We established an efficient strategy to direct human pluripotent stem cells, including human embryonic stem cells (hESCs) and an induced pluripotent stem cell (iPSC) line derived from patients with cystic fibrosis, to differentiate into pancreatic ductal epithelial cells (PDECs). After purification, more than 98% of hESC-derived PDECs expressed functional cystic fibrosis transmembrane conductance regulator (CFTR) protein. In addition, iPSC lines were derived from a patient with CF carrying compound frameshift and mRNA splicing mutations and were differentiated to PDECs. PDECs derived from Weill Cornell cystic fibrosis (WCCF)-iPSCs showed defective expression of mature CFTR protein and impaired chloride ion channel activity, recapitulating functional defects of patients with CF at the cellular level. These studies provide a new methodology to derive pure PDECs expressing CFTR and establish a "disease in a dish" platform to identify drug candidates to rescue the pancreatic defects of patients with CF.
Significance: An efficient strategy was established to direct human pluripotent stem cells, including human embryonic stem cells (hESCs) and an induced pluripotent stem cell line derived from patients with cystic fibrosis (CF-iPSCs), to differentiate into pancreatic ductal epithelial cells (PDECs). After purification, more than 98% of hESC-PDECs derived from CF-iPSCs showed defective expression of mature cystic fibrosis transmembrane conductance regulator (CFTR) protein and impaired chloride ion channel activity, recapitulating functional pancreatic defects of patients with CF at the cellular level. These studies provide a new methodology for deriving pure PDECs expressing CFTR, and they establish a "disease-in-a-dish" platform for identifying drug candidates to rescue the pancreatic defects of these patients.
Keywords: Chloride ion efflux assay; Directed differentiation; Pancreatic defects of cystic fibrosis; Reprogramming.
©AlphaMed Press.
Figures




References
-
- Wilke M, Buijs-Offerman RM, Aarbiou J, et al. Mouse models of cystic fibrosis: Phenotypic analysis and research applications. J Cyst Fibros. 2011;10(suppl 2):S152–S171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

