An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model
- PMID: 27034505
- PMCID: PMC4840298
- DOI: 10.1101/gad.275891.115
An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model
Abstract
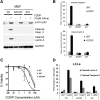
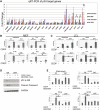
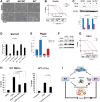
A nonsynonymous single-nucleotide polymorphism at codon 47 in TP53 exists in African-descent populations (P47S, rs1800371; referred to here as S47). Here we report that, in human cell lines and a mouse model, the S47 variant exhibits a modest decrease in apoptosis in response to most genotoxic stresses compared with wild-type p53 but exhibits a significant defect in cell death induced by cisplatin. We show that, compared with wild-type p53, S47 has nearly indistinguishable transcriptional function but shows impaired ability to transactivate a subset of p53 target genes, including two involved in metabolism:Gls2(glutaminase 2) and Sco2 We also show that human and mouse cells expressing the S47 variant are markedly resistant to cell death by agents that induce ferroptosis (iron-mediated nonapoptotic cell death). We show that mice expressing S47 in homozygous or heterozygous form are susceptible to spontaneous cancers of diverse histological types. Our data suggest that the S47 variant may contribute to increased cancer risk in individuals of African descent, and our findings highlight the need to assess the contribution of this variant to cancer risk in these populations. These data also confirm the potential relevance of metabolism and ferroptosis to tumor suppression by p53.
Keywords: Ser46 phosphorylation; ferroptosis; metabolism; p53; polymorphism; tumor suppression.
© 2016 Jennis et al.; Published by Cold Spring Harbor Laboratory Press.
Figures




References
-
- Dixon SJ, Stockwell BR. 2014. The role of iron and reactive oxygen species in cell death. Nat Chem Biol 10: 9–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
