Engineering of a high lipid producing Yarrowia lipolytica strain
- PMID: 27034715
- PMCID: PMC4815080
- DOI: 10.1186/s13068-016-0492-3
Engineering of a high lipid producing Yarrowia lipolytica strain
Abstract
Background: Microbial lipids are produced by many oleaginous organisms including the well-characterized yeast Yarrowia lipolytica, which can be engineered for increased lipid yield by up-regulation of the lipid biosynthetic pathway and down-regulation or deletion of competing pathways.
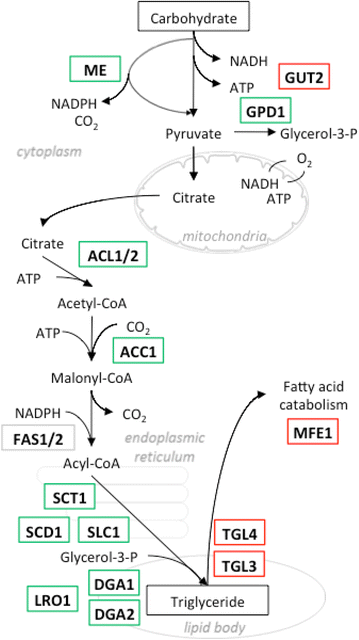
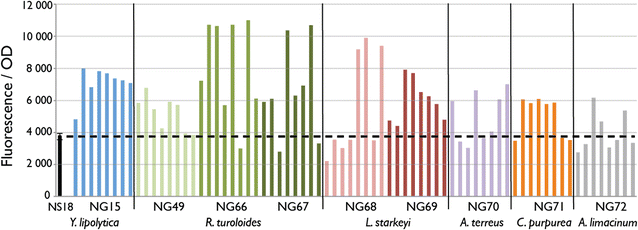
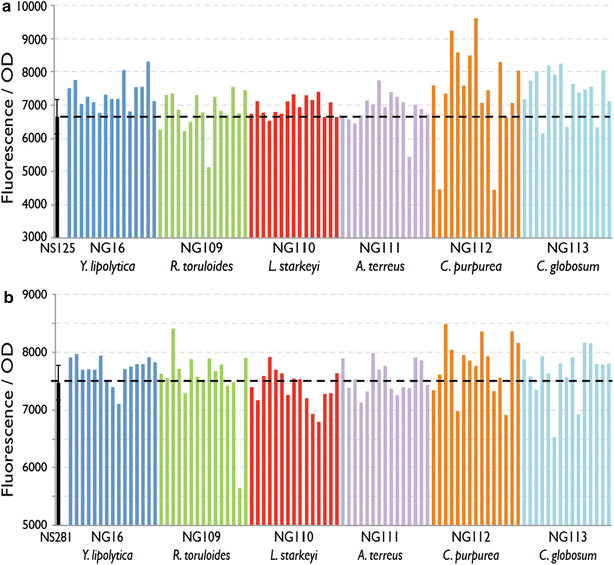
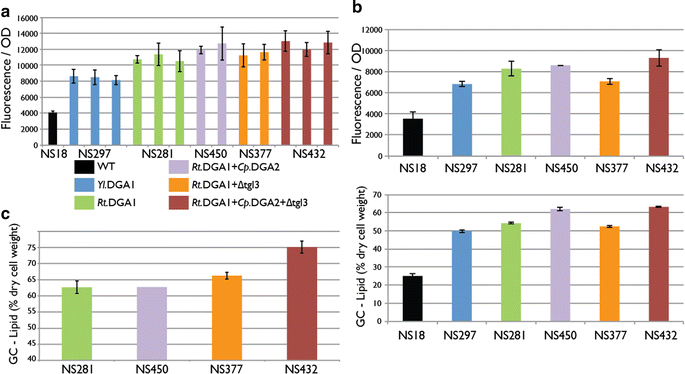
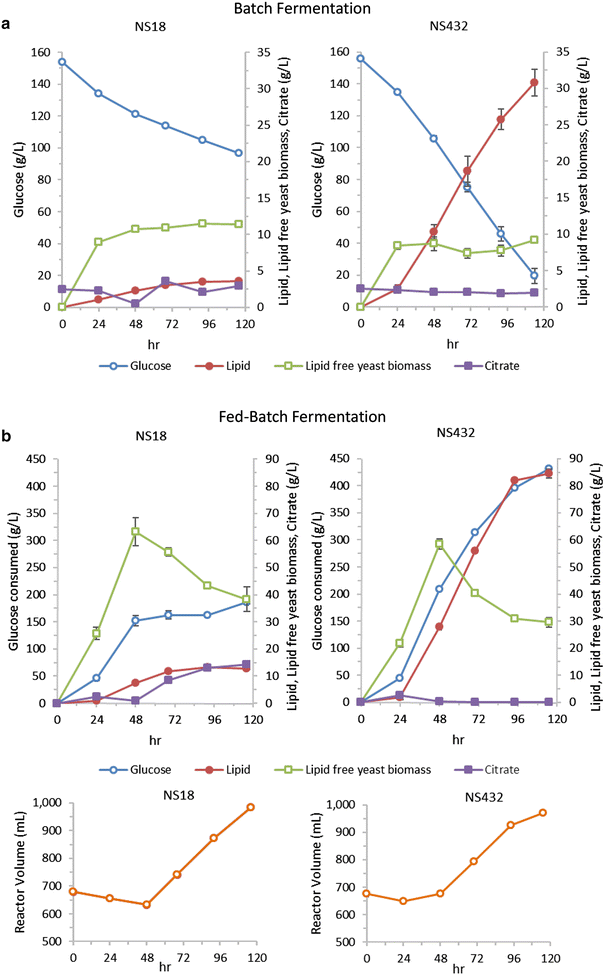
Results: We describe a strain engineering strategy centered on diacylglycerol acyltransferase (DGA) gene overexpression that applied combinatorial screening of overexpression and deletion genetic targets to construct a high lipid producing yeast biocatalyst. The resulting strain, NS432, combines overexpression of a heterologous DGA1 enzyme from Rhodosporidium toruloides, a heterlogous DGA2 enzyme from Claviceps purpurea, and deletion of the native TGL3 lipase regulator. These three genetic modifications, selected for their effect on lipid production, enabled a 77 % lipid content and 0.21 g lipid per g glucose yield in batch fermentation. In fed-batch glucose fermentation NS432 produced 85 g/L lipid at a productivity of 0.73 g/L/h.
Conclusions: The yields, productivities, and titers reported in this study may further support the applied goal of cost-effective, large -scale microbial lipid production for use as biofuels and biochemicals.
Keywords: Lipid accumulation; Metabolic engineering; Oleaginous yeast; Yarrowia lipolytica.
Figures
Similar articles
-
Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production.Metab Eng. 2013 Jan;15:1-9. doi: 10.1016/j.ymben.2012.08.007. Epub 2012 Sep 28. Metab Eng. 2013. PMID: 23026119
-
Developing cellulolytic Yarrowia lipolytica as a platform for the production of valuable products in consolidated bioprocessing of cellulose.Biotechnol Biofuels. 2018 May 15;11:141. doi: 10.1186/s13068-018-1144-6. eCollection 2018. Biotechnol Biofuels. 2018. PMID: 29785208 Free PMC article.
-
Functional overexpression and characterization of lipogenesis-related genes in the oleaginous yeast Yarrowia lipolytica.Appl Microbiol Biotechnol. 2016 Apr;100(8):3781-98. doi: 10.1007/s00253-016-7376-0. Epub 2016 Feb 26. Appl Microbiol Biotechnol. 2016. PMID: 26915993
-
Oleaginous yeasts: Promising platforms for the production of oleochemicals and biofuels.Biotechnol Bioeng. 2017 Sep;114(9):1915-1920. doi: 10.1002/bit.26337. Epub 2017 May 29. Biotechnol Bioeng. 2017. PMID: 28498495 Review.
-
Holistic Approaches in Lipid Production by Yarrowia lipolytica.Trends Biotechnol. 2018 Nov;36(11):1157-1170. doi: 10.1016/j.tibtech.2018.06.007. Epub 2018 Jul 11. Trends Biotechnol. 2018. PMID: 30006239 Review.
Cited by
-
Engineering of Yarrowia lipolytica for production of astaxanthin.Synth Syst Biotechnol. 2017 Oct 20;2(4):287-294. doi: 10.1016/j.synbio.2017.10.002. eCollection 2017 Dec. Synth Syst Biotechnol. 2017. PMID: 29552653 Free PMC article.
-
The metabolism and genetic regulation of lipids in the oleaginous yeast Yarrowia lipolytica.Braz J Microbiol. 2019 Jan;50(1):23-31. doi: 10.1007/s42770-018-0004-7. Epub 2018 Nov 29. Braz J Microbiol. 2019. PMID: 30637631 Free PMC article. Review.
-
Could termites be hiding a goldmine of obscure yet promising yeasts for energy crisis solutions based on aromatic wastes? A critical state-of-the-art review.Biotechnol Biofuels Bioprod. 2022 Apr 4;15(1):35. doi: 10.1186/s13068-022-02131-z. Biotechnol Biofuels Bioprod. 2022. PMID: 35379342 Free PMC article. Review.
-
Production, Biosynthesis, and Commercial Applications of Fatty Acids From Oleaginous Fungi.Front Nutr. 2022 May 19;9:873657. doi: 10.3389/fnut.2022.873657. eCollection 2022. Front Nutr. 2022. PMID: 35694158 Free PMC article. Review.
-
Single cell assessment of yeast metabolic engineering for enhanced lipid production using Raman and AFM-IR imaging.Biotechnol Biofuels. 2018 Apr 10;11:106. doi: 10.1186/s13068-018-1108-x. eCollection 2018. Biotechnol Biofuels. 2018. PMID: 29643936 Free PMC article.
References
-
- Donot F, Fontana A, Baccou JC, Strub C, Schorr-Galindo S. Single cell oils (SCOs) from oleaginous yeasts and moulds: production and genetics. Biomass Bioenergy. 2014;68:135–150. doi: 10.1016/j.biombioe.2014.06.016. - DOI
-
- Bati N, Hammond EG, Glatz BA. Biomodification of fats and oils: trials with Candida lipolytica. J Am Oil Chem Soc. 1984;61:1743–1746. doi: 10.1007/BF02582139. - DOI
-
- Papanikolaou S, Chevalot I, Galiotou-Panayotou M, Komaitis M, Marc I, Aggelis G. Industrial derivative of tallow: a promising renewable substrate for microbial lipid, single-cell protein and lipase production by Yarrowia lipolytica. Electron J Biotechn. 2007;10:425–435.
LinkOut - more resources
Full Text Sources
Other Literature Sources

