Band 3 Erythrocyte Membrane Protein Acts as Redox Stress Sensor Leading to Its Phosphorylation by p (72) Syk
- PMID: 27034738
- PMCID: PMC4806680
- DOI: 10.1155/2016/6051093
Band 3 Erythrocyte Membrane Protein Acts as Redox Stress Sensor Leading to Its Phosphorylation by p (72) Syk
Abstract
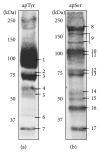
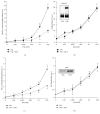
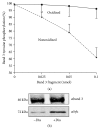
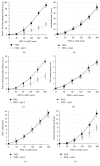
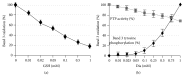
In erythrocytes, the regulation of the redox sensitive Tyr phosphorylation of band 3 and its functions are still partially defined. A role of band 3 oxidation in regulating its own phosphorylation has been previously suggested. The current study provides evidences to support this hypothesis: (i) in intact erythrocytes, at 2 mM concentration of GSH, band 3 oxidation, and phosphorylation, Syk translocation to the membrane and Syk phosphorylation responded to the same micromolar concentrations of oxidants showing identical temporal variations; (ii) the Cys residues located in the band 3 cytoplasmic domain are 20-fold more reactive than GSH; (iii) disulfide linked band 3 cytoplasmic domain docks Syk kinase; (iv) protein Tyr phosphatases are poorly inhibited at oxidant concentrations leading to massive band 3 oxidation and phosphorylation. We also observed that hemichromes binding to band 3 determined its irreversible oxidation and phosphorylation, progressive hemolysis, and serine hyperphosphorylation of different cytoskeleton proteins. Syk inhibitor suppressed the phosphorylation of band 3 also preventing serine phosphorylation changes and hemolysis. Our data suggest that band 3 acts as redox sensor regulating its own phosphorylation and that hemichromes leading to the protracted phosphorylation of band 3 may trigger a cascade of events finally leading to hemolysis.
Figures

References
-
- Arese P., Mannuzzu L., Turrini F. Pathophysiology of favism. Folia Haematologica. 1989;116(5):745–752. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

