Study of Valproic Acid-Enhanced Hepatocyte Steatosis
- PMID: 27034954
- PMCID: PMC4789392
- DOI: 10.1155/2016/9576503
Study of Valproic Acid-Enhanced Hepatocyte Steatosis
Abstract
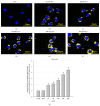
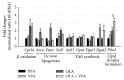
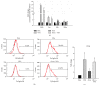
Valproic acid (VPA) is one of the most widely used antiepilepsy drugs. However, several side effects, including weight gain and fatty liver, have been reported in patients following VPA treatment. In this study, we explored the molecular mechanisms of VPA-induced hepatic steatosis using FL83B cell line-based in vitro model. Using fluorescent lipid staining technique, we found that VPA enhanced oleic acid- (OLA-) induced lipid accumulation in a dose-dependent manner in hepatocytes; this may be due to upregulated lipid uptake, triacylglycerol (TAG) synthesis, and lipid droplet formation. Real-time PCR results showed that, following VPA treatment, the expression levels of genes encoding cluster of differentiation 36 (Cd36), low-density lipoprotein receptor-related protein 1 (Lrp1), diacylglycerol acyltransferase 2 (Dgat2), and perilipin 2 (Plin2) were increased, that of carnitine palmitoyltransferase I a (Cpt1a) was not affected, and those of acetyl-Co A carboxylase α (Acca) and fatty acid synthase (Fasn) were decreased. Furthermore, using immunofluorescence staining and flow cytometry analyses, we found that VPA also induced peroxisome proliferator-activated receptor γ (PPARγ) nuclear translocation and increased levels of cell-surface CD36. Based on these results, we propose that VPA may enhance OLA-induced hepatocyte steatosis through the upregulation of PPARγ- and CD36-dependent lipid uptake, TAG synthesis, and lipid droplet formation.
Figures


References
-
- Saleh D. A. A., Ismail M. A., Ibrahim A. M. Non alcoholic fatty liver disease, insulin resistance, dyslipidemia and atherogenic ratios in epileptic children and adolescents on long term antiepileptic drug therapy. Pakistan Journal of Biological Sciences. 2012;15(2):68–77. doi: 10.3923/pjbs.2012.68.77. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

