MicroRNA-29b inhibits TGF-β1-induced fibrosis via regulation of the TGF-β1/Smad pathway in primary human endometrial stromal cells
- PMID: 27035110
- PMCID: PMC4838148
- DOI: 10.3892/mmr.2016.5062
MicroRNA-29b inhibits TGF-β1-induced fibrosis via regulation of the TGF-β1/Smad pathway in primary human endometrial stromal cells
Abstract
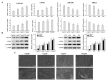
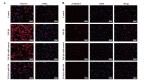
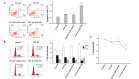
Transforming growth factor (TGF)‑β1 has a key role in the regulation of fibrosis and organ dysfunction. During the pathogenesis and progression of vital organ fibrosis, the microRNA (miR)‑29 family is irregularly downregulated and exogenous supplementation of miR‑29b has a strong anti‑fibrotic capacity. However, whether TGF‑β1 is able to provoke endometrial fibrosis, and the role of miR‑29 in endometrial fibrosis remain unclear. In the present study, RT‑qPCR, immunocytochemistry, western blot analysis, scanning electron microscopy, immunofluorescence staining, cell proliferation assay and flow cytometric analysis were employed. The results demonstrated that the expression levels of collagen, type 1, alpha 1 (COL1A1), α‑smooth muscle actin (α‑SMA) and phosphorylated (p)‑Smad2/3 were increased, whereas miR‑29b and maternally expressed gene 3 (MEG3) were decreased in primary endometrial stromal cells (ESCs) in response to TGF‑β1 stimulation, in a time and dose‑dependent manner. Furthermore, overexpression of miR‑29b markedly reduced the expression levels of COL1A1 and α‑SMA, and decreased the expression and nuclear accumulation of p‑Smad2/3. In addition, ectopic overexpression of miR‑29b increased the expression levels of MEG3, inhibited myofibroblast‑like cell proliferation and induced apoptosis. These findings indicated that miR‑29b may have a significant anti‑fibrotic role, and may attenuate TGF‑β1‑induced fibrosis in ESCs. Therefore, exogenous miR‑29b may serve as a potential therapeutic agent for the treatment of endometrial fibrosis.
Figures


Similar articles
-
LncRNA MALAT1 Knockdown Alleviates Fibrogenic Response in Human Endometrial Stromal Cells Via the miR-22-3p/TGFβR1/Smad2/3 Pathway.Cell Biochem Biophys. 2024 Dec;82(4):3573-3584. doi: 10.1007/s12013-024-01445-z. Epub 2024 Aug 17. Cell Biochem Biophys. 2024. PMID: 39154131
-
Quercetin regulates fibrogenic responses of endometrial stromal cell by upregulating miR-145 and inhibiting the TGF-β1/Smad2/Smad3 pathway.Acta Histochem. 2020 Oct;122(7):151600. doi: 10.1016/j.acthis.2020.151600. Epub 2020 Aug 29. Acta Histochem. 2020. PMID: 33066828
-
MicroRNA‑326 inhibits endometrial fibrosis by regulating TGF‑β1/Smad3 pathway in intrauterine adhesions.Mol Med Rep. 2018 Aug;18(2):2286-2292. doi: 10.3892/mmr.2018.9187. Epub 2018 Jun 19. Mol Med Rep. 2018. PMID: 29956752
-
LncRNA HOTAIR promotes endometrial fibrosis by activating TGF-β1/Smad pathway.Acta Biochim Biophys Sin (Shanghai). 2020 Dec 29;52(12):1337-1347. doi: 10.1093/abbs/gmaa120. Acta Biochim Biophys Sin (Shanghai). 2020. PMID: 33313721
-
Connective tissue growth factor mediates transforming growth factor β-induced collagen expression in human endometrial stromal cells.PLoS One. 2019 Jan 29;14(1):e0210765. doi: 10.1371/journal.pone.0210765. eCollection 2019. PLoS One. 2019. PMID: 30695033 Free PMC article.
Cited by
-
CircRNA_0263 and circRNA_1507 are dysregulated in a rat model of atrial fibrosis induced by chronic intermittent hypoxia.Am J Transl Res. 2023 Jan 15;15(1):63-81. eCollection 2023. Am J Transl Res. 2023. PMID: 36777857 Free PMC article.
-
tRNA‑derived small RNAs: A novel class of small RNAs in human hypertrophic scar fibroblasts.Int J Mol Med. 2020 Jan;45(1):115-130. doi: 10.3892/ijmm.2019.4411. Epub 2019 Nov 25. Int J Mol Med. 2020. PMID: 31939611 Free PMC article.
-
MicroRNA as novel biomarkers and therapeutic targets in diabetic kidney disease: An update.FASEB Bioadv. 2019 Apr 10;1(6):375-388. doi: 10.1096/fba.2018-00064. eCollection 2019 Jun. FASEB Bioadv. 2019. PMID: 32123840 Free PMC article.
-
Long noncoding RNA MEG3 suppresses liver cancer cells growth through inhibiting β-catenin by activating PKM2 and inactivating PTEN.Cell Death Dis. 2018 Feb 15;9(3):253. doi: 10.1038/s41419-018-0305-7. Cell Death Dis. 2018. PMID: 29449541 Free PMC article.
-
TNFα-induced altered miRNA expression links to NF-κB signaling pathway in endometriosis.Res Sq [Preprint]. 2023 May 4:rs.3.rs-2870585. doi: 10.21203/rs.3.rs-2870585/v1. Res Sq. 2023. Update in: Inflammation. 2023 Dec;46(6):2055-2070. doi: 10.1007/s10753-023-01862-x. PMID: 37205467 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

