The Tyrosine Kinase Inhibitor Sunitinib Affects Ovulation but Not Ovarian Reserve in Mouse: A Preclinical Study
- PMID: 27035144
- PMCID: PMC4818017
- DOI: 10.1371/journal.pone.0152872
The Tyrosine Kinase Inhibitor Sunitinib Affects Ovulation but Not Ovarian Reserve in Mouse: A Preclinical Study
Abstract
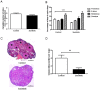
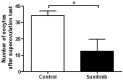
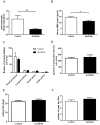
The aim of the study was to evaluate ovarian toxicity of tyrosine kinase inhibitor (TKI) sunitinib, since only scarce data are available on gonadal function after this treatment. Six-week-old female mice received orally, once daily, vehicle or sunitinib (50 mg/kg/d) during 5 weeks. Fertility parameters were analyzed from ovulation to litter assessment. Sunitinib exposure significantly reduced (i) corpora lutea number per ovary (1.1 ± 0.38 in sunitinib group versus 4 ± 0.79 in control group, p<0.01) and (ii) serum Anti Müllerian hormone (AMH) levels in sunitinib treated mice (12.01 ± 1.16) compared to control mice (14.33 ± 0.87 ng/ml, p< 0.05). However, primordial and growing follicles numbers per ovary were not different in both groups. After treatment withdrawal, female mice in both groups were able to obtain litters. These data could be helpful to counsel clinicians and patients, when fertility preservation methods are discussed, before TKI treatment in girls and young women.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

