Suppression of STAT5b in pancreatic cancer cells leads to attenuated gemcitabine chemoresistance, adhesion and invasion
- PMID: 27035235
- PMCID: PMC4869940
- DOI: 10.3892/or.2016.4727
Suppression of STAT5b in pancreatic cancer cells leads to attenuated gemcitabine chemoresistance, adhesion and invasion
Abstract
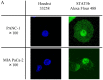
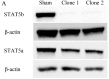
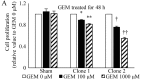
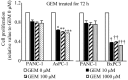
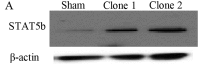
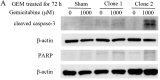
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal solid malignancies, and there is an urgent need for new therapeutic strategies based on the molecular biology of PDAC. Signal transducers and activators of transcription 5 (STAT5) are known to be activated in a variety of malignancies and involved in tumor proliferation, apoptosis, and invasion, whereas the expression and biological role of STAT5b in PDAC are less clearly defined. In the present study, we examined the expression and role of STAT5b in human pancreatic cancer cell lines. Expressions of STAT5b mRNA and protein were detected in eight kinds of pancreatic cancer cells. Confocal microscopy and western blot analysis indicated that STAT5b is localized in both cytoplasm and nuclei. Immunoprecipitation analysis revealed tyrosine phosphorylation of STAT5b in pancreatic cancer cells. These results indicate that STAT5b in pancreatic cancer cells is constitutively activated. STAT5b shRNA clones in PANC-1 cells, which express relatively high levels of STAT5b, exhibited reduced chemoresistance against gemcitabine, adhesion and invasion compared to sham. On the other hand, AsPC-1 and BxPC3 cells, which express relatively low levels of STAT5b, exhibited reduced chemoresistance compared to PANC-1 cells. Moreover, STAT5b overexpression clones in AsPC-1 cells exhibited increased chemoresistance compared to sham. STAT5b shRNA clones in PANC-1 cells were more sensitive to the proapoptotic actions of gemcitabine, as evidenced by PARP and cleaved caspase-3 activation. Gemcitabine also significantly reduced Bcl-xL levels in the STAT5b shRNA-expressing cells. We also investigated the clinicopathological characteristics of STAT5b expression of PDAC. Although a significant correlation between STAT5b expression and overall survival rates was not observed, a significant correlation with main pancreatic duct invasion was observed. These findings suggest that STAT5b confers gemcitabine chemoresistance and promotes cell adherence and invasiveness in pancreatic cancer cells. Targeting STAT5b may lead to novel therapeutic strategies for PDAC.
Figures
















References
-
- Burris HA, III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

