Endometrial Stromal Decidualization Responds Reversibly to Hormone Stimulation and Withdrawal
- PMID: 27035651
- PMCID: PMC4891781
- DOI: 10.1210/en.2015-1942
Endometrial Stromal Decidualization Responds Reversibly to Hormone Stimulation and Withdrawal
Abstract
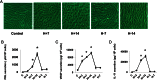
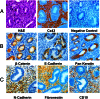
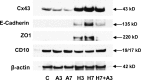
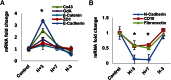
Human endometrial stromal decidualization is required for embryo receptivity, angiogenesis, and placentation. Previous studies from our laboratories established that connexin (Cx)-43 critically regulates endometrial stromal cell (ESC) differentiation, whereas gap junction blockade prevents it. The current study evaluated the plasticity of ESC morphology and Cx43 expression, as well as other biochemical markers of cell differentiation, in response to decidualizing hormones. Primary human ESC cultures were exposed to 10 nM estradiol, 100 nM progesterone, and 0.5 mM cAMP for up to 14 days, followed by hormone withdrawal for 14 days, mimicking a biphasic ovulatory cycle. Reversible differentiation was documented by characteristic changes in cell shape. Cx43 was reversibly up- and down-regulated after the estradiol, progesterone, and cAMP treatment and withdrawal, respectively, paralleled by fluctuations in prolactin, vascular endothelial growth factor, IL-11, and glycodelin secretion. Markers of mesenchymal-epithelial transition (MET), and its counterpart epithelial-mesenchymal transition, followed reciprocal patterns corresponding to the morphological changes. Incubation in the presence of 18α-glycyrrhetinic acid, an inhibitor of gap junctions, partially reversed the expression of decidualization and MET markers. In the absence of hormones, Cx43 overexpression promoted increases in vascular endothelial growth factor and IL-11 secretion, up-regulated MET markers, and reduced N-cadherin, an epithelial-mesenchymal transition marker. The combined results support the hypothesis that Cx43-containing gap junctions and endocrine factors cooperate to regulate selected biomarkers of stromal decidualization and MET and suggest roles for both phenomena in endometrial preparation for embryonic receptivity.
Figures




References
-
- Martin RD. The evolution of human reproduction: a primatological perspective. Am J Phys Anthropol Suppl. 2007;45:59–84. - PubMed
-
- Gargett CE, Masuda H. Adult stem cells in the endometrium. Mol Hum Reprod. 2010;16:818–834. - PubMed
-
- Brosens JJ, Wilson MS, Lam EW. FOXO transcription factors: from cell fate decisions to regulation of human female reproduction. Adv Exp Med Biol. 2009;665:227–241. - PubMed
-
- Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–453. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

