Extracellular vesicles and infectious diseases: new complexity to an old story
- PMID: 27035809
- PMCID: PMC4811125
- DOI: 10.1172/JCI81132
Extracellular vesicles and infectious diseases: new complexity to an old story
Abstract
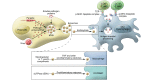
Exosomes and other extracellular microvesicles (ExMVs) have important functions in intercellular communication and regulation. During the course of infection, these vesicles can convey pathogen molecules that serve as antigens or agonists of innate immune receptors to induce host defense and immunity, or that serve as regulators of host defense and mediators of immune evasion. These molecules may include proteins, nucleic acids, lipids, and carbohydrates. Pathogen molecules may be disseminated by incorporation into vesicles that are created and shed by host cells, or they may be incorporated into vesicles shed from microbial cells. Involvement of ExMVs in the induction of immunity and host defense is widespread among many pathogens, whereas their involvement in immune evasion mechanisms is prominent among pathogens that establish chronic infection and is found in some that cause acute infection. Because of their immunogenicity and enrichment of pathogen molecules, exosomes may also have potential in vaccine preparations and as diagnostic markers. Additionally, the ability of exosomes to deliver molecules to recipient cells raises the possibility of their use for drug/therapy delivery. Thus, ExMVs play a major role in the pathogenesis of infection and provide exciting potential for the development of novel diagnostic and therapeutic approaches.
Figures

Similar articles
-
Extracellular vesicles: masters of intercellular communication and potential clinical interventions.J Clin Invest. 2016 Apr 1;126(4):1139-43. doi: 10.1172/JCI87316. Epub 2016 Apr 1. J Clin Invest. 2016. PMID: 27035805 Free PMC article. Review.
-
Regulation of chronic inflammatory and immune processes by extracellular vesicles.J Clin Invest. 2016 Apr 1;126(4):1173-80. doi: 10.1172/JCI81131. Epub 2016 Apr 1. J Clin Invest. 2016. PMID: 27035808 Free PMC article. Review.
-
Procoagulant and immunogenic properties of melanoma exosomes, microvesicles and apoptotic vesicles.Oncotarget. 2016 Aug 30;7(35):56279-56294. doi: 10.18632/oncotarget.10783. Oncotarget. 2016. PMID: 27462921 Free PMC article.
-
Host derived exosomes-pathogens interactions: Potential functions of exosomes in pathogen infection.Biomed Pharmacother. 2018 Dec;108:1451-1459. doi: 10.1016/j.biopha.2018.09.174. Epub 2018 Oct 8. Biomed Pharmacother. 2018. PMID: 30372847 Review.
-
Exosomes: From Functions in Host-Pathogen Interactions and Immunity to Diagnostic and Therapeutic Opportunities.Rev Physiol Biochem Pharmacol. 2016;172:39-75. doi: 10.1007/112_2016_7. Rev Physiol Biochem Pharmacol. 2016. PMID: 27600934 Review.
Cited by
-
The role of Mycobacterium tuberculosis exosomal miRNAs in host pathogen cross-talk as diagnostic and therapeutic biomarkers.Front Microbiol. 2024 Aug 8;15:1441781. doi: 10.3389/fmicb.2024.1441781. eCollection 2024. Front Microbiol. 2024. PMID: 39176271 Free PMC article. Review.
-
The complex, bidirectional role of extracellular vesicles in infection.Biochem Soc Trans. 2021 Apr 30;49(2):881-891. doi: 10.1042/BST20200788. Biochem Soc Trans. 2021. PMID: 33860784 Free PMC article. Review.
-
Exosomes as New Generation Vehicles for Drug Delivery: Biomedical Applications and Future Perspectives.Molecules. 2022 Oct 27;27(21):7289. doi: 10.3390/molecules27217289. Molecules. 2022. PMID: 36364116 Free PMC article. Review.
-
Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia.Thorax. 2019 Jan;74(1):43-50. doi: 10.1136/thoraxjnl-2018-211576. Epub 2018 Aug 3. Thorax. 2019. PMID: 30076187 Free PMC article.
-
Evaluation of EV Storage Buffer for Efficient Preservation of Engineered Extracellular Vesicles.Int J Mol Sci. 2023 Aug 16;24(16):12841. doi: 10.3390/ijms241612841. Int J Mol Sci. 2023. PMID: 37629020 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

