A ring closing metathesis strategy for carbapyranosides of xylose and arabinose
- PMID: 27035910
- PMCID: PMC5137374
- DOI: 10.1016/j.carres.2016.03.002
A ring closing metathesis strategy for carbapyranosides of xylose and arabinose
Abstract
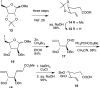
The synthesis of β-carba-xylo and arabino pyranosides of cholestanol is described. The synthetic strategy, which is analogous to the Postema approach to C-glycosides, centers on the ring closing metathesis of an enol ether-alkene precursor to give a cyclic enol ether that is elaborated to a carba-pyranoside via hydroboration-oxidation on the olefin. The method, which is attractive for its modularity and stereoselectivity, may find wider applications to carba-hexopyranosides and other complex cycloalkyl ether frameworks.
Keywords: Arabinose; Carbasugar; Cholestanol (PubChem CID: 6665); Cycloalkyl ether; Dimethyl sulfide borane (PubChem CID: 9833925); Glycomimetic; Grubbs catalyst 2nd generation (PubChem CID: 11147261); Methyl (triphenylphosphoranylidene)acetate (PubChem CID: 17453); Methyl 2,3-O-isopropylidene-beta-d-ribofuranoside (PubChem CID: 96666); Methyl alpha-d-arabinofuranoside (PubChem CID: 11389582); RCM; Tebbe reagent (PubChem CID: 91617563); Xylose.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
References
-
- La Ferla B, Airoldi C, Zona C, Orsato A, Cardona F, Merlo S, Sironi E, D'Orazio G, Nicotra F. Nat. Prod. Rep. 2011;28:630–648. - PubMed
-
- Wojtkielewicz A, D1ugosz M, Maj J, Morzycki JW, Nowakowski M, Renkiewicz J, Strnad M, Swaczynová J, Wilczewska AZ, Wójcik J. J. Med. Chem. 2007;50:3667–3673. - PubMed
-
- Lorent JH, Quetin-Leclercq J, Mingeot-Leclercq M-P. Org. Biomol. Chem. 2014;12:8803–8022. - PubMed
-
- Shukla RK, Tiwari A. Crit. Rev. Ther. Drug Carrier Syst. 2011;28:255–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources