Modulation of nitrogen vacancy charge state and fluorescence in nanodiamonds using electrochemical potential
- PMID: 27035935
- PMCID: PMC4839455
- DOI: 10.1073/pnas.1504451113
Modulation of nitrogen vacancy charge state and fluorescence in nanodiamonds using electrochemical potential
Abstract
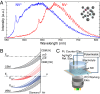
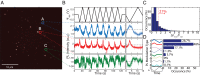
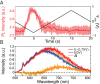
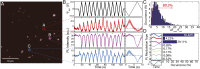
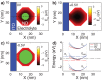
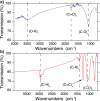
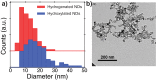
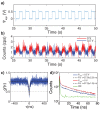
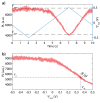
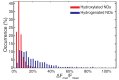
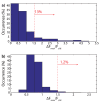
The negatively charged nitrogen vacancy (NV(-)) center in diamond has attracted strong interest for a wide range of sensing and quantum information processing applications. To this end, recent work has focused on controlling the NV charge state, whose stability strongly depends on its electrostatic environment. Here, we demonstrate that the charge state and fluorescence dynamics of single NV centers in nanodiamonds with different surface terminations can be controlled by an externally applied potential difference in an electrochemical cell. The voltage dependence of the NV charge state can be used to stabilize the NV(-) state for spin-based sensing protocols and provides a method of charge state-dependent fluorescence sensing of electrochemical potentials. We detect clear NV fluorescence modulation for voltage changes down to 100 mV, with a single NV and down to 20 mV with multiple NV centers in a wide-field imaging mode. These results suggest that NV centers in nanodiamonds could enable parallel optical detection of biologically relevant electrochemical potentials.
Keywords: fluorescence microscopy; nanodiamond; nitrogen vacancy center; voltage indicator; voltage sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Stimulated emission depletion microscopy resolves individual nitrogen vacancy centers in diamond nanocrystals.ACS Nano. 2013 Dec 23;7(12):10912-9. doi: 10.1021/nn404421b. Epub 2013 Nov 23. ACS Nano. 2013. PMID: 24245613
-
Advances in Stabilization and Enrichment of Shallow Nitrogen-Vacancy Centers in Diamond for Biosensing and Spin-Polarization Transfer.Biosensors (Basel). 2023 Jun 29;13(7):691. doi: 10.3390/bios13070691. Biosensors (Basel). 2023. PMID: 37504090 Free PMC article. Review.
-
A Label-Free Diamond Microfluidic DNA Sensor Based on Active Nitrogen-Vacancy Center Charge State Control.ACS Appl Mater Interfaces. 2021 Apr 28;13(16):18500-18510. doi: 10.1021/acsami.1c01118. Epub 2021 Apr 13. ACS Appl Mater Interfaces. 2021. PMID: 33849273
-
Fluorescent nanodiamonds for luminescent thermometry in the biological transparency window.Opt Lett. 2018 Jul 15;43(14):3317-3320. doi: 10.1364/OL.43.003317. Opt Lett. 2018. PMID: 30004495
-
Nanoscale quantum sensing with Nitrogen-Vacancy centers in nanodiamonds - A magnetic resonance perspective.Prog Nucl Magn Reson Spectrosc. 2023 Apr-Jun;134-135:20-38. doi: 10.1016/j.pnmrs.2022.12.001. Epub 2022 Dec 13. Prog Nucl Magn Reson Spectrosc. 2023. PMID: 37321756 Review.
Cited by
-
Fluorescent Reporters for Sensing Membrane Potential: Tools for Bioelectricity.Bioelectricity. 2022 May 26;4(2):108-116. doi: 10.1089/bioe.2022.0017. eCollection 2022 May. Bioelectricity. 2022. PMID: 39350779 Free PMC article. No abstract available.
-
Manipulating solid-state spin concentration through charge transport.Proc Natl Acad Sci U S A. 2023 Aug 8;120(32):e2305621120. doi: 10.1073/pnas.2305621120. Epub 2023 Aug 1. Proc Natl Acad Sci U S A. 2023. PMID: 37527342 Free PMC article.
-
Free radical detection in precision-cut mouse liver slices with diamond-based quantum sensing.Proc Natl Acad Sci U S A. 2024 Oct 22;121(43):e2317921121. doi: 10.1073/pnas.2317921121. Epub 2024 Oct 14. Proc Natl Acad Sci U S A. 2024. PMID: 39401360 Free PMC article.
-
Monitoring spin coherence of single nitrogen-vacancy centers in nanodiamonds during pH changes in aqueous buffer solutions.RSC Adv. 2019 Apr 23;9(22):12606-12614. doi: 10.1039/c9ra02282a. eCollection 2019 Apr 17. RSC Adv. 2019. PMID: 35515823 Free PMC article.
-
Ion sensors with crown ether-functionalized nanodiamonds.ArXiv [Preprint]. 2023 Jan 9:arXiv:2301.03143v1. ArXiv. 2023. PMID: 36713238 Free PMC article. Preprint.
References
-
- Tamarat P, et al. Stark shift control of single optical centers in diamond. Phys Rev Lett. 2006;97(8):083002. - PubMed
-
- Bassett LC, Heremans FJ, Yale CG, Buckley BB, Awschalom DD. Electrical tuning of single nitrogen-vacancy center optical transitions enhanced by photoinduced fields. Phys Rev Lett. 2011;107(26):266403. - PubMed
-
- Dolde F, et al. Electric-field sensing using single diamond spins. Nat Phys. 2011;7(6):459–463.
-
- Maze JR, et al. Nanoscale magnetic sensing with an individual electronic spin in diamond. Nature. 2008;455(7213):644–647. - PubMed
-
- Gaebel T, et al. Photochromism in single nitrogen-vacancy defect in diamond. Appl Phys B. 2006;82(2):243–246.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

