GOLDEN 2-LIKE transcription factors for chloroplast development affect ozone tolerance through the regulation of stomatal movement
- PMID: 27035938
- PMCID: PMC4839443
- DOI: 10.1073/pnas.1513093113
GOLDEN 2-LIKE transcription factors for chloroplast development affect ozone tolerance through the regulation of stomatal movement
Abstract
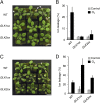
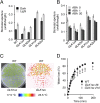
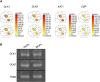
Stomatal movements regulate gas exchange, thus directly affecting the efficiency of photosynthesis and the sensitivity of plants to air pollutants such as ozone. The GARP family transcription factors GOLDEN 2-LIKE1 (GLK1) and GLK2 have known functions in chloroplast development. Here, we show that Arabidopsis thaliana (A. thaliana) plants expressing the chimeric repressors for GLK1 and -2 (GLK1/2-SRDX) exhibited a closed-stomata phenotype and strong tolerance to ozone. By contrast, plants that overexpress GLK1/2 exhibited an open-stomata phenotype and higher sensitivity to ozone. The plants expressing GLK1-SRDX had reduced expression of the genes for inwardly rectifying K(+) (K(+) in) channels and reduced K(+) in channel activity. Abscisic acid treatment did not affect the stomatal phenotype of 35S:GLK1/2-SRDX plants or the transcriptional activity for K(+) in channel gene, indicating that GLK1/2 act independently of abscisic acid signaling. Our results indicate that GLK1/2 positively regulate the expression of genes for K(+) in channels and promote stomatal opening. Because the chimeric GLK1-SRDX repressor driven by a guard cell-specific promoter induced a closed-stomata phenotype without affecting chloroplast development in mesophyll cells, modulating GLK1/2 activity may provide an effective tool to control stomatal movements and thus to confer resistance to air pollutants.
Keywords: K+in channel; ozone; repressor; stomatal movement; transcription factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
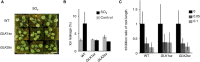






Similar articles
-
GOLDEN2-LIKE Transcription Factors Regulate WRKY40 Expression in Response to Abscisic Acid.Plant Physiol. 2019 Apr;179(4):1844-1860. doi: 10.1104/pp.18.01466. Epub 2019 Feb 5. Plant Physiol. 2019. PMID: 30723180 Free PMC article.
-
bHLH transcription factors that facilitate K⁺ uptake during stomatal opening are repressed by abscisic acid through phosphorylation.Sci Signal. 2013 Jun 18;6(280):ra48. doi: 10.1126/scisignal.2003760. Sci Signal. 2013. PMID: 23779086
-
Disruption of a gene encoding C4-dicarboxylate transporter-like protein increases ozone sensitivity through deregulation of the stomatal response in Arabidopsis thaliana.Plant Cell Physiol. 2008 Jan;49(1):2-10. doi: 10.1093/pcp/pcm174. Epub 2007 Dec 15. Plant Cell Physiol. 2008. PMID: 18084014
-
The guard cell as a single-cell model towards understanding drought tolerance and abscisic acid action.J Exp Bot. 2009;60(5):1439-63. doi: 10.1093/jxb/ern340. Epub 2009 Jan 30. J Exp Bot. 2009. PMID: 19181866 Review.
-
The plant stomatal lineage at a glance.J Cell Sci. 2019 Apr 26;132(8):jcs228551. doi: 10.1242/jcs.228551. J Cell Sci. 2019. PMID: 31028153 Free PMC article. Review.
Cited by
-
Antagonistic modules regulate photosynthesis-associated nuclear genes via GOLDEN2-LIKE transcription factors.Plant Physiol. 2022 Mar 28;188(4):2308-2324. doi: 10.1093/plphys/kiab600. Plant Physiol. 2022. PMID: 34951648 Free PMC article.
-
Molecular Evolution and Genetic Variation of G2-Like Transcription Factor Genes in Wheat (Triticum aestivum L.).Genes (Basel). 2023 Jun 26;14(7):1341. doi: 10.3390/genes14071341. Genes (Basel). 2023. PMID: 37510246 Free PMC article.
-
GOLDEN2-LIKE Transcription Factors Regulate WRKY40 Expression in Response to Abscisic Acid.Plant Physiol. 2019 Apr;179(4):1844-1860. doi: 10.1104/pp.18.01466. Epub 2019 Feb 5. Plant Physiol. 2019. PMID: 30723180 Free PMC article.
-
Blue Light Regulation of Stomatal Opening and the Plasma Membrane H+-ATPase.Plant Physiol. 2017 Jun;174(2):531-538. doi: 10.1104/pp.17.00166. Epub 2017 May 2. Plant Physiol. 2017. PMID: 28465463 Free PMC article. Review.
-
Maize GOLDEN2-LIKE genes enhance biomass and grain yields in rice by improving photosynthesis and reducing photoinhibition.Commun Biol. 2020 Apr 1;3(1):151. doi: 10.1038/s42003-020-0887-3. Commun Biol. 2020. PMID: 32238902 Free PMC article.
References
-
- Pinto DM, Blande JD, Souza SR, Nerg AM, Holopainen JK. Plant volatile organic compounds (VOCs) in ozone (O3) polluted atmospheres: The ecological effects. J Chem Ecol. 2010;36(1):22–34. - PubMed
-
- Ludwikow A, Sadowski J. Gene networks in plant ozone stress response and tolerance. J Integr Plant Biol. 2008;50(10):1256–1267. - PubMed
-
- Wilkinson S, Mills G, Illidge R, Davies WJ. How is ozone pollution reducing our food supply? J Exp Bot. 2012;63(2):527–536. - PubMed
-
- Kangasjarvi J, et al. Signalling and cell death in ozone-exposed plants. Plant Cell Environ. 2005;28(8):1021–1036.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

