Comprehensive mutagenesis of the fimS promoter regulatory switch reveals novel regulation of type 1 pili in uropathogenic Escherichia coli
- PMID: 27035967
- PMCID: PMC4839427
- DOI: 10.1073/pnas.1522958113
Comprehensive mutagenesis of the fimS promoter regulatory switch reveals novel regulation of type 1 pili in uropathogenic Escherichia coli
Abstract
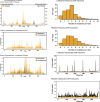
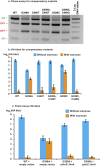
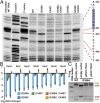
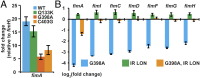
Type 1 pili (T1P) are major virulence factors for uropathogenic Escherichia coli (UPEC), which cause both acute and recurrent urinary tract infections. T1P expression therefore is of direct relevance for disease. T1P are phase variable (both piliated and nonpiliated bacteria exist in a clonal population) and are controlled by an invertible DNA switch (fimS), which contains the promoter for the fim operon encoding T1P. Inversion of fimS is stochastic but may be biased by environmental conditions and other signals that ultimately converge at fimS itself. Previous studies of fimS sequences important for T1P phase variation have focused on laboratory-adapted E coli strains and have been limited in the number of mutations or by alteration of the fimS genomic context. We surmounted these limitations by using saturating genomic mutagenesis of fimS coupled with accurate sequencing to detect both mutations and phase status simultaneously. In addition to the sequences known to be important for biasing fimS inversion, our method also identifies a previously unknown pair of 5' UTR inverted repeats that act by altering the relative fimA levels to control phase variation. Thus we have uncovered an additional layer of T1P regulation potentially impacting virulence and the coordinate expression of multiple pilus systems.
Keywords: fimS; phase variation; saturating chromosomal mutagenesis; type 1 pili; urinary tract infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Comment in
-
Re: Comprehensive Mutagenesis of the FimS Promoter Regulatory Switch Reveals Novel Regulation of Type 1 Pili in Uropathogenic Escherichia coli.J Urol. 2017 Jun;197(6):1463-1464. doi: 10.1016/j.juro.2017.03.015. Epub 2017 Mar 11. J Urol. 2017. PMID: 28505911 No abstract available.
Similar articles
-
Comprehensive Identification of Fim-Mediated Inversions in Uropathogenic Escherichia coli with Structural Variation Detection Using Relative Entropy.mSphere. 2019 Apr 10;4(2):e00693-18. doi: 10.1128/mSphere.00693-18. mSphere. 2019. PMID: 30971446 Free PMC article.
-
Human Urine Decreases Function and Expression of Type 1 Pili in Uropathogenic Escherichia coli.mBio. 2015 Jun 30;6(4):e00820. doi: 10.1128/mBio.00820-15. mBio. 2015. PMID: 26126855 Free PMC article.
-
Altered Regulation of the Diguanylate Cyclase YaiC Reduces Production of Type 1 Fimbriae in a Pst Mutant of Uropathogenic Escherichia coli CFT073.J Bacteriol. 2017 Nov 14;199(24):e00168-17. doi: 10.1128/JB.00168-17. Print 2017 Dec 15. J Bacteriol. 2017. PMID: 28924030 Free PMC article.
-
What Flips the Switch? Signals and Stress Regulating Extraintestinal Pathogenic Escherichia coli Type 1 Fimbriae (Pili).Microorganisms. 2021 Dec 21;10(1):5. doi: 10.3390/microorganisms10010005. Microorganisms. 2021. PMID: 35056454 Free PMC article. Review.
-
The regulation of pap and type 1 fimbriation in Escherichia coli.Adv Microb Physiol. 2001;45:1-49. doi: 10.1016/s0065-2911(01)45001-6. Adv Microb Physiol. 2001. PMID: 11450107 Review.
Cited by
-
Comprehensive Identification of Fim-Mediated Inversions in Uropathogenic Escherichia coli with Structural Variation Detection Using Relative Entropy.mSphere. 2019 Apr 10;4(2):e00693-18. doi: 10.1128/mSphere.00693-18. mSphere. 2019. PMID: 30971446 Free PMC article.
-
Genome-wide detection of conservative site-specific recombination in bacteria.PLoS Genet. 2018 Apr 5;14(4):e1007332. doi: 10.1371/journal.pgen.1007332. eCollection 2018 Apr. PLoS Genet. 2018. PMID: 29621238 Free PMC article.
-
Taxonomic and functional analyses reveal existence of virulence and antibiotic resistance genes in beach sand bacterial populations.Arch Microbiol. 2021 May;203(4):1753-1766. doi: 10.1007/s00203-020-02165-7. Epub 2021 Jan 20. Arch Microbiol. 2021. PMID: 33474608 Free PMC article.
-
Unveiling a Novel Mechanism of Enhanced Secretion, Cargo Loading, and Accelerated Dynamics of Bacterial Extracellular Vesicles Following Antibiotic Exposure.J Extracell Vesicles. 2025 Jul;14(7):e70131. doi: 10.1002/jev2.70131. J Extracell Vesicles. 2025. PMID: 40704571 Free PMC article.
-
Systematic approach for dissecting the molecular mechanisms of transcriptional regulation in bacteria.Proc Natl Acad Sci U S A. 2018 May 22;115(21):E4796-E4805. doi: 10.1073/pnas.1722055115. Epub 2018 May 4. Proc Natl Acad Sci U S A. 2018. PMID: 29728462 Free PMC article.
References
-
- Foxman B, Brown P. Epidemiology of urinary tract infections: Transmission and risk factors, incidence, and costs. Infect Dis Clin North Am. 2003;17(2):227–241. - PubMed
-
- Mulvey MA, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282(5393):1494–1497. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

