A bacterial Argonaute with noncanonical guide RNA specificity
- PMID: 27035975
- PMCID: PMC4839417
- DOI: 10.1073/pnas.1524385113
A bacterial Argonaute with noncanonical guide RNA specificity
Abstract
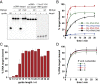
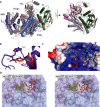
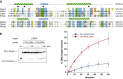
Eukaryotic Argonaute proteins induce gene silencing by small RNA-guided recognition and cleavage of mRNA targets. Although structural similarities between human and prokaryotic Argonautes are consistent with shared mechanistic properties, sequence and structure-based alignments suggested that Argonautes encoded within CRISPR-cas [clustered regularly interspaced short palindromic repeats (CRISPR)-associated] bacterial immunity operons have divergent activities. We show here that the CRISPR-associated Marinitoga piezophila Argonaute (MpAgo) protein cleaves single-stranded target sequences using 5'-hydroxylated guide RNAs rather than the 5'-phosphorylated guides used by all known Argonautes. The 2.0-Å resolution crystal structure of an MpAgo-RNA complex reveals a guide strand binding site comprising residues that block 5' phosphate interactions. Using structure-based sequence alignment, we were able to identify other putative MpAgo-like proteins, all of which are encoded within CRISPR-cas loci. Taken together, our data suggest the evolution of an Argonaute subclass with noncanonical specificity for a 5'-hydroxylated guide.
Keywords: Argonaute; RNA interference; small noncoding RNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Programmable RNA recognition using a CRISPR-associated Argonaute.Proc Natl Acad Sci U S A. 2018 Mar 27;115(13):3368-3373. doi: 10.1073/pnas.1717725115. Epub 2018 Mar 12. Proc Natl Acad Sci U S A. 2018. PMID: 29531059 Free PMC article.
-
DNA recognition by an RNA-guided bacterial Argonaute.PLoS One. 2017 May 17;12(5):e0177097. doi: 10.1371/journal.pone.0177097. eCollection 2017. PLoS One. 2017. PMID: 28520746 Free PMC article.
-
STRUCTURAL BIOLOGY. A Cas9-guide RNA complex preorganized for target DNA recognition.Science. 2015 Jun 26;348(6242):1477-81. doi: 10.1126/science.aab1452. Science. 2015. PMID: 26113724
-
Type III CRISPR-Cas System: Introduction And Its Application for Genetic Manipulations.Curr Issues Mol Biol. 2018;26:1-14. doi: 10.21775/cimb.026.001. Epub 2017 Sep 7. Curr Issues Mol Biol. 2018. PMID: 28879852 Review.
-
Argonaute proteins: structures and their endonuclease activity.Mol Biol Rep. 2021 May;48(5):4837-4849. doi: 10.1007/s11033-021-06476-w. Epub 2021 Jun 11. Mol Biol Rep. 2021. PMID: 34117606 Review.
Cited by
-
Catalytically active prokaryotic Argonautes employ phospholipase D family proteins to strengthen immunity against different genetic invaders.mLife. 2024 Sep 4;3(3):403-416. doi: 10.1002/mlf2.12138. eCollection 2024 Sep. mLife. 2024. PMID: 39359674 Free PMC article.
-
The prokaryotic Argonaute proteins enhance homology sequence-directed recombination in bacteria.Nucleic Acids Res. 2019 Apr 23;47(7):3568-3579. doi: 10.1093/nar/gkz040. Nucleic Acids Res. 2019. PMID: 30698806 Free PMC article.
-
Systematic prediction of genes functionally linked to CRISPR-Cas systems by gene neighborhood analysis.Proc Natl Acad Sci U S A. 2018 Jun 5;115(23):E5307-E5316. doi: 10.1073/pnas.1803440115. Epub 2018 May 21. Proc Natl Acad Sci U S A. 2018. PMID: 29784811 Free PMC article.
-
Argonaute proteins: Structural features, functions and emerging roles.J Adv Res. 2020 Apr 29;24:317-324. doi: 10.1016/j.jare.2020.04.017. eCollection 2020 Jul. J Adv Res. 2020. PMID: 32455006 Free PMC article. Review.
-
Specific targeting of plasmids with Argonaute enables genome editing.Nucleic Acids Res. 2023 May 8;51(8):4086-4099. doi: 10.1093/nar/gkad191. Nucleic Acids Res. 2023. PMID: 36987855 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

