Host-parasite oscillation dynamics and evolution in a compartmentalized RNA replication system
- PMID: 27035976
- PMCID: PMC4839452
- DOI: 10.1073/pnas.1524404113
Host-parasite oscillation dynamics and evolution in a compartmentalized RNA replication system
Abstract
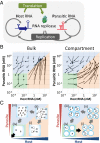
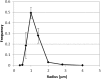
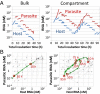
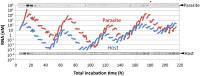
To date, various cellular functions have been reconstituted in vitro such as self-replication systems using DNA, RNA, and proteins. The next important challenges include the reconstitution of the interactive networks of self-replicating species and investigating how such interactions generate complex ecological behaviors observed in nature. Here, we synthesized a simple replication system composed of two self-replicating host and parasitic RNA species. We found that the parasitic RNA eradicates the host RNA under bulk conditions; however, when the system is compartmentalized, a continuous oscillation pattern in the population dynamics of the two RNAs emerges. The oscillation pattern changed as replication proceeded mainly owing to the evolution of the host RNA. These results demonstrate that a cell-like compartment plays an important role in host-parasite ecological dynamics and suggest that the origin of the host-parasite coevolution might date back to the very early stages of the evolution of life.
Keywords: RNA replication; compartment; evolution; oscillation; parasite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

