Individual variability in human blood metabolites identifies age-related differences
- PMID: 27036001
- PMCID: PMC4843419
- DOI: 10.1073/pnas.1603023113
Individual variability in human blood metabolites identifies age-related differences
Abstract
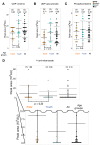
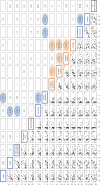
Metabolites present in human blood document individual physiological states influenced by genetic, epigenetic, and lifestyle factors. Using high-resolution liquid chromatography-mass spectrometry (LC-MS), we performed nontargeted, quantitative metabolomics analysis in blood of 15 young (29 ± 4 y of age) and 15 elderly (81 ± 7 y of age) individuals. Coefficients of variation (CV = SD/mean) were obtained for 126 blood metabolites of all 30 donors. Fifty-five RBC-enriched metabolites, for which metabolomics studies have been scarce, are highlighted here. We found 14 blood compounds that show remarkable age-related increases or decreases; they include 1,5-anhydroglucitol, dimethyl-guanosine, acetyl-carnosine, carnosine, ophthalmic acid, UDP-acetyl-glucosamine,N-acetyl-arginine,N6-acetyl-lysine, pantothenate, citrulline, leucine, isoleucine, NAD(+), and NADP(+) Six of them are RBC-enriched, suggesting that RBC metabolomics is highly valuable for human aging research. Age differences are partly explained by a decrease in antioxidant production or increasing inefficiency of urea metabolism among the elderly. Pearson's coefficients demonstrated that some age-related compounds are correlated, suggesting that aging affects them concomitantly. Although our CV values are mostly consistent with those CVs previously published, we here report previously unidentified CVs of 51 blood compounds. Compounds having moderate to high CV values (0.4-2.5) are often modified. Compounds having low CV values, such as ATP and glutathione, may be related to various diseases because their concentrations are strictly controlled, and changes in them would compromise health. Thus, human blood is a rich source of information about individual metabolic differences.
Keywords: CV value; aging markers; antioxidants; red blood cells; urea cycle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Comment in
-
QnAs with Mitsuhiro Yanagida.Proc Natl Acad Sci U S A. 2016 Jun 14;113(24):6581-2. doi: 10.1073/pnas.1607589113. Epub 2016 May 31. Proc Natl Acad Sci U S A. 2016. PMID: 27247401 Free PMC article. No abstract available.
-
Metabolomics of aging requires large-scale longitudinal studies with replication.Proc Natl Acad Sci U S A. 2016 Jun 21;113(25):E3470. doi: 10.1073/pnas.1607062113. Epub 2016 Jun 14. Proc Natl Acad Sci U S A. 2016. PMID: 27303027 Free PMC article. No abstract available.
References
-
- Rapoport SM, Schewe T, Thiele B-J. Maturational breakdown of mitochondria and other organelles in reticulocytes. In: Harris JR, editor. Erythroid Cells. Springer; New York: 1990. pp. 151–194.
-
- van Wijk R, van Solinge WW. The energy-less red blood cell is lost: Erythrocyte enzyme abnormalities of glycolysis. Blood. 2005;106(13):4034–4042. - PubMed
-
- Bax BE, Bain MD, Talbot PJ, Parker-Williams EJ, Chalmers RA. Survival of human carrier erythrocytes in vivo. Clin Sci (Lond) 1999;96(2):171–178. - PubMed
-
- Chaleckis R, et al. Unexpected similarities between the Schizosaccharomyces and human blood metabolomes, and novel human metabolites. Mol Biosyst. 2014;10(10):2538–2551. - PubMed
-
- Fernie AR, Trethewey RN, Krotzky AJ, Willmitzer L. Metabolite profiling: From diagnostics to systems biology. Nat Rev Mol Cell Biol. 2004;5(9):763–769. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

