Myofibroblasts are distinguished from activated skin fibroblasts by the expression of AOC3 and other associated markers
- PMID: 27036009
- PMCID: PMC4839407
- DOI: 10.1073/pnas.1603534113
Myofibroblasts are distinguished from activated skin fibroblasts by the expression of AOC3 and other associated markers
Abstract
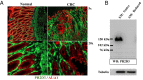
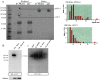
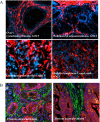
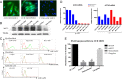
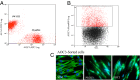
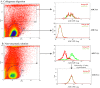
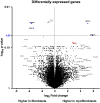
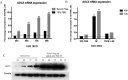
Pericryptal myofibroblasts in the colon and rectum play an important role in regulating the normal colorectal stem cell niche and facilitating tumor progression. Myofibroblasts previously have been distinguished from normal fibroblasts mostly by the expression of α smooth muscle actin (αSMA). We now have identified AOC3 (amine oxidase, copper containing 3), a surface monoamine oxidase, as a new marker of myofibroblasts by showing that it is the target protein of the myofibroblast-reacting mAb PR2D3. The normal and tumor tissue distribution and the cell line reactivity of AOC3 match that expected for myofibroblasts. We have shown that the surface expression of AOC3 is sensitive to digestion by trypsin and collagenase and that anti-AOC3 antibodies can be used for FACS sorting of myofibroblasts obtained by nonenzymatic procedures. Whole-genome microarray mRNA-expression profiles of myofibroblasts and skin fibroblasts revealed four additional genes that are significantly differentially expressed in these two cell types: NKX2-3 and LRRC17 in myofibroblasts and SHOX2 and TBX5 in skin fibroblasts. TGFβ substantially down-regulated AOC3 expression in myofibroblasts but in skin fibroblasts it dramatically increased the expression of αSMA. A knockdown of NKX2-3 in myofibroblasts caused a decrease of myofibroblast-related gene expression and increased expression of the fibroblast-associated gene SHOX2, suggesting that NKX2-3 is a key mediator for maintaining myofibroblast characteristics. Our results show that colorectal myofibroblasts, as defined by the expression of AOC3, NKX2-3, and other markers, are a distinctly different cell type from TGFβ-activated fibroblasts.
Keywords: AOC3; NKX2-3; myofibroblasts; tumor microenvironment; α smooth muscle actin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Identification of AOC3 and LRRC17 as Colonic Fibroblast Activation Markers and Their Potential Roles in Colorectal Cancer Progression.Asian Pac J Cancer Prev. 2023 Sep 1;24(9):3099-3107. doi: 10.31557/APJCP.2023.24.9.3099. Asian Pac J Cancer Prev. 2023. PMID: 37774061 Free PMC article.
-
Amine oxidase activity regulates the development of pulmonary fibrosis.FASEB J. 2017 Jun;31(6):2477-2491. doi: 10.1096/fj.201600935R. Epub 2017 Mar 1. FASEB J. 2017. PMID: 28251930
-
NG2/CSPG4, CD146/MCAM and VAP1/AOC3 are regulated by myocardin-related transcription factors in smooth muscle cells.Sci Rep. 2021 Mar 16;11(1):5955. doi: 10.1038/s41598-021-85335-x. Sci Rep. 2021. PMID: 33727640 Free PMC article.
-
Nkx2.5/Csx represses myofibroblast differentiation.Am J Respir Cell Mol Biol. 2010 Feb;42(2):218-26. doi: 10.1165/rcmb.2008-0404OC. Epub 2009 Apr 24. Am J Respir Cell Mol Biol. 2010. PMID: 19395679 Free PMC article.
-
Amine oxidase copper-containing 3 (AOC3) inhibition: a potential novel target for the management of diabetic retinopathy.Int J Retina Vitreous. 2021 Apr 12;7(1):30. doi: 10.1186/s40942-021-00288-7. Int J Retina Vitreous. 2021. PMID: 33845913 Free PMC article. Review.
Cited by
-
Differential Effects of the Absence of Nkx2-3 and MAdCAM-1 on the Distribution of Intestinal Type 3 Innate Lymphoid Cells and Postnatal SILT Formation in Mice.Front Immunol. 2019 Mar 5;10:366. doi: 10.3389/fimmu.2019.00366. eCollection 2019. Front Immunol. 2019. PMID: 30891037 Free PMC article.
-
In search of definitions: Cancer-associated fibroblasts and their markers.Int J Cancer. 2020 Feb 15;146(4):895-905. doi: 10.1002/ijc.32193. Epub 2019 Feb 28. Int J Cancer. 2020. PMID: 30734283 Free PMC article. Review.
-
Role of cancer-associated fibroblasts in the resistance to antitumor therapy, and their potential therapeutic mechanisms in non-small cell lung cancer.Oncol Lett. 2021 May;21(5):413. doi: 10.3892/ol.2021.12674. Epub 2021 Mar 23. Oncol Lett. 2021. PMID: 33841574 Free PMC article. Review.
-
Single-cell sequencing highlights heterogeneity and malignant progression in actinic keratosis and cutaneous squamous cell carcinoma.Elife. 2023 Dec 15;12:e85270. doi: 10.7554/eLife.85270. Elife. 2023. PMID: 38099574 Free PMC article.
-
Expression of Amine Oxidase Proteins in Adrenal Cortical Neoplasm and Pheochromocytoma.Biomedicines. 2023 Jul 4;11(7):1896. doi: 10.3390/biomedicines11071896. Biomedicines. 2023. PMID: 37509535 Free PMC article.
References
-
- Donnellan WL. The structure of the colonic mucosa. The epithelium and subepithelial reticulohistiocytic complex. Gastroenterology. 1965;49(5):496–514. - PubMed
-
- Kaye GI, Lane N, Pascal RR. Colonic pericryptal fibroblast sheath: Replication, migration, and cytodifferentiation of a mesenchymal cell system in adult tissue. II. Fine structural aspects of normal rabbit and human colon. Gastroenterology. 1968;54(5):852–865. - PubMed
-
- Pascal RR, Kaye GI, Lane N. Colonic pericryptal fibroblast sheath: Replication, migration, and cytodifferentiation of a mesenchymal cell system in adult tissue. I. Autoradiographic studies of normal rabbit colon. Gastroenterology. 1968;54(5):835–851. - PubMed
-
- Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27(5):549–550. - PubMed
-
- Majno G, Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR. Contraction of granulation tissue in vitro: Similarity to smooth muscle. Science. 1971;173(3996):548–550. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

