Acthar gel in the treatment of nephrotic syndrome: a multicenter retrospective case series
- PMID: 27036111
- PMCID: PMC4815175
- DOI: 10.1186/s12882-016-0241-7
Acthar gel in the treatment of nephrotic syndrome: a multicenter retrospective case series
Abstract
Background: Current first-line anti-proteinuric treatments for nephrotic syndrome (NS) do not produce an effective response in all patients and are not tolerated by some patients. Additional effective and tolerable treatment options in NS are strongly needed. This retrospective case series is the largest to date to examine Acthar gel (adrenocorticotropic hormone, ACTH) in patients with varied-etiology NS.
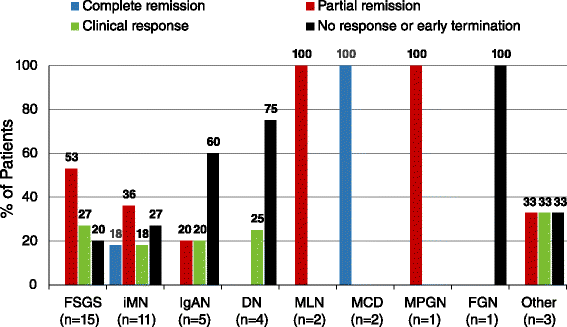
Methods: This multicenter retrospective case series included adult patients with NS (N = 44) treated with Acthar gel at 6 clinical practices. NS etiologies included idiopathic focal segmental glomerulosclerosis (FSGS, 15), idiopathic membranous nephropathy (iMN, 11), IgA nephropathy (IgAN, 5), diabetic nephropathy (DN, 4), systemic lupus erythematosus class V membranous lupus nephritis (MLN, 2), minimal change disease (MCD, 2), membranoproliferative glomerulonephritis (MPGN, 1), fibrillary glomerulonephritis (FGN, 1), and unbiopsied NS (3). Proteinuria response was assessed as percent reduction from baseline and percent of patients meeting complete remission (final proteinuria <500 mg/d), partial remission (≥50 % reduction in proteinuria from baseline and final proteinuria 500-3500 mg/d), clinical response (≥30 % reduction in proteinuria from baseline that did not meet criteria for complete or partial remission), and no response (failed to meet remission or clinical response criteria) following Acthar gel therapy. Safety and tolerability were examined using adverse event (AE) frequency reported by patients or treating nephrologists and frequency of early discontinuation of treatment due to AEs.
Results: 68.2 % (30/44) of patients had received prior NS treatment with immunosuppressive or cytotoxic therapies. Thirty-seven patients completed Acthar gel treatment. Seven patients (15.9 %) had early termination due to AEs, including weight gain (2), hypertension (2), edema (1), fatigue (1), seizures (1) and for reasons not stated (2). Proteinuria reduction ≥30 % was shown in 81.1 % (30/37) of patients and 62.2 % (23/37) showed ≥50 % proteinuria reduction. Proteinuria responses were greatest in MCD (n = 2/2 complete remission), MLN (n = 2/2 partial remission), MPGN (n = 1/1 partial remission), FSGS (n = 12/15 [80.0 %] partial remission or clinical response), and iMN (n = 8/11 [72.7 %] complete remission, partial remission, or clinical response).
Conclusions: Acthar gel may meet an important treatment need in patients with treatment-resistant NS in response to first-line therapies, patients unable to tolerate first-line therapies, and in patients with advanced disease.
Keywords: ACTH; Acthar gel; Nephrotic syndrome; Proteinuria.
Figures
References
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl. 2012;2:139–274. doi: 10.1038/kisup.2012.9. - DOI
-
- Segarra-Medrano A, Jatem-Escalante E, Agraz-Pamplona I, Carnicer-Caceres C, Ramos-Terrades N, Ostos-Roldan E, Quiles-Perez MT, Arbos-Via MA. Treatment of idiopathic focal segmental glomerulosclerosis: options in the event of resistance to corticosteroids and calcineurin inhibitors. Nefrologia. 2013;33:448–461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

