Pathological tau deposition in Motor Neurone Disease and frontotemporal lobar degeneration associated with TDP-43 proteinopathy
- PMID: 27036121
- PMCID: PMC4818389
- DOI: 10.1186/s40478-016-0301-z
Pathological tau deposition in Motor Neurone Disease and frontotemporal lobar degeneration associated with TDP-43 proteinopathy
Abstract
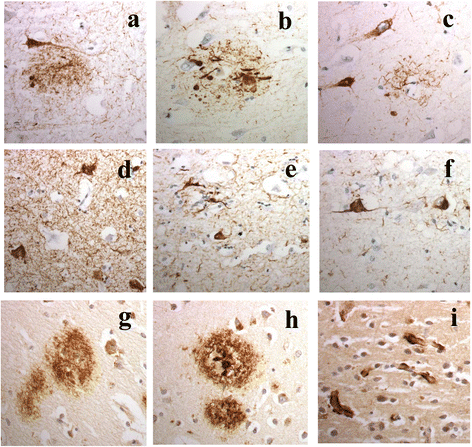
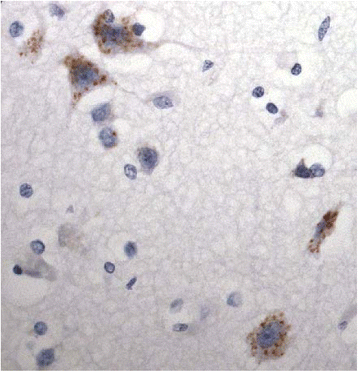
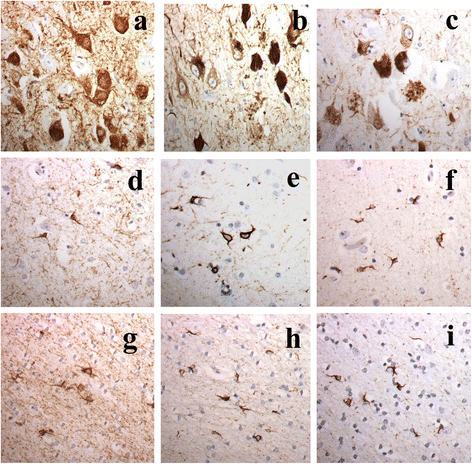
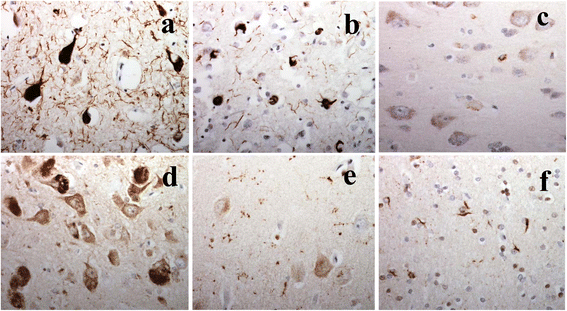
It has been suggested that patients with motor neurone disease (MND) and those with MND combined with behavioural variant frontotemporal dementia (bvFTD) (ie FTD + MND) or with FTD alone might exist on a continuum based on commonalities of neuropathology and/or genetic risk. Moreover, it has been reported that both a neuronal and a glial cell tauopathy can accompany the TDP-43 proteinopathy in patients with motor neurone disease (MND) with cognitive changes, and that the tauopathy may be fundamental to disease pathogenesis and clinical phenotype. In the present study, we sought to substantiate these latter findings, and test this concept of a pathological continuum, in a consecutive series of 41 patients with MND, 16 with FTD + MND and 23 with FTD without MND. Paraffin sections of frontal, entorhinal, temporal and occipital cortex and hippocampus were immunostained for tau pathology using anti-tau antibodies, AT8, pThr(175) and pThr(217), and for amyloid β protein (Aβ) using 4G8 antibody. Twenty four (59 %) patients with MND, 7 (44 %) patients with FTD + MND and 10 (43 %) patients with FTD showed 'significant' tau pathology (ie more than just an isolated neurofibrillary tangle or a few neuropil threads in one or more brain regions examined). In most instances, this bore the histological characteristics of an Alzheimer's disease process involving entorhinal cortex, hippocampus, temporal cortex, frontal cortex and occipital cortex in decreasing frequency, accompanied by a deposition of Aβ up to Thal phase 3, though 2 patients with MND, and 1 with FTD did show tau pathology beyond Braak stage III. Four other patients with MND showed novel neuronal tau pathology, within the frontal cortex alone, specifically detected by pThr(175) antibody, which was characterised by a fine granular or more clumped aggregation of tau without neurofibrillary tangles or neuropil threads. However, none of these 4 patients had clinically evident cognitive disorder, and this type of tau pathology was not seen in any of the FTD + MND or FTD patients. Finally, two patients, one with MND and one with FTD, showed a tau pathology consistent with Argyrophilic Grain Disease (AGD). Western blotting and use of 3- and 4-repeat tau antibodies confirmed the histological interpretation of Alzheimer's disease type pathology in all instances except for those patients with accompanying AGD where a banding pattern on western blot, and immunohistochemistry, confirmed 4-repeat tauopathy. In all 3 patient groups, amyloid pathology was more likely to be present in patients dying after 65 years of age, and in the presence of APOE ε4 allele. We conclude that tau pathological changes are equally common amongst patients with MND, FTD + MND and FTD though, in most instances, these are limited in extent. In patients with MND, when cognitive impairment is present this is most likely due to an accompanying/evolving (coincidental) Alzheimer's disease process or, as in a single case, Dementia with Lewy bodies, within the cerebral cortex rather than as a result of TDP-43 proteinopathy. Conversely, in FTD and FTD + MND dementia is more likely to be associated with TDP-43 proteinopathy than tau. Hence, present study shows no progression in severity of (tau) pathology from MND through FTD + MND to FTD, and does not support the concept of these conditions forming a continuum of clinical or pathological change.
Figures
References
-
- Abrahams S, Goldstein LH, Kew JJ, Brooks DJ, Lloyd CM, Frith CD, Leigh PN. Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET Stud Brain. 1996;119:2105–2120. - PubMed
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

