Towards a standardised informed consent procedure for live donor nephrectomy: the PRINCE (Process of Informed Consent Evaluation) project-study protocol for a nationwide prospective cohort study
- PMID: 27036141
- PMCID: PMC4823441
- DOI: 10.1136/bmjopen-2015-010594
Towards a standardised informed consent procedure for live donor nephrectomy: the PRINCE (Process of Informed Consent Evaluation) project-study protocol for a nationwide prospective cohort study
Abstract
Introduction: Informed consent is mandatory for all (surgical) procedures, but it is even more important when it comes to living kidney donors undergoing surgery for the benefit of others. Donor education, leading to informed consent, needs to be carried out according to certain standards. Informed consent procedures for live donor nephrectomy vary per centre, and even per individual healthcare professional. The basis for a standardised, uniform surgical informed consent procedure for live donor nephrectomy can be created by assessing what information donors need to hear to prepare them for the operation and convalescence.
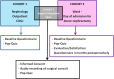
Methods and analysis: The PRINCE (Process of Informed Consent Evaluation) project is a prospective, multicentre cohort study, to be carried out in all eight Dutch kidney transplant centres. Donor knowledge of the procedure and postoperative course will be evaluated by means of pop quizzes. A baseline cohort (prior to receiving any information from a member of the transplant team in one of the transplant centres) will be compared with a control group, the members of which receive the pop quiz on the day of admission for donor nephrectomy. Donor satisfaction will be evaluated for all donors who completed the admission pop-quiz. The primary end point is donor knowledge. In addition, those elements that have to be included in the standardised format informed consent procedure will be identified. Secondary end points are donor satisfaction, current informed consent practices in the different centres (eg, how many visits, which personnel, what kind of information is disclosed, in which format, etc) and correlation of donor knowledge with surgeons' estimation thereof.
Ethics and dissemination: Approval for this study was obtained from the medical ethical committee of the Erasmus MC, University Medical Center, Rotterdam, on 18 February 2015. Secondary approval has been obtained from the local ethics committees in six participating centres. Approval in the last centre has been sought.
Results: Outcome will be published in a scientific journal.
Trial registration number: NTR5374; Pre-results.
Keywords: TRANSPLANT SURGERY.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
Similar articles
-
Living Kidney Donor Knowledge of Provided Information and Informed Consent: The PRINCE Study.J Clin Med. 2022 Jan 28;11(3):698. doi: 10.3390/jcm11030698. J Clin Med. 2022. PMID: 35160147 Free PMC article.
-
Towards a standardized informed consent procedure for live donor nephrectomy: What do surgeons tell their donors?Int J Surg. 2016 Aug;32:83-8. doi: 10.1016/j.ijsu.2016.05.063. Epub 2016 Jun 1. Int J Surg. 2016. PMID: 27260313
-
Ethical issues surrounding high-risk kidney recipients: implications for the living donor.Prog Transplant. 2007 Sep;17(3):180-2. doi: 10.1177/152692480701700304. Prog Transplant. 2007. PMID: 17944156 Review.
-
Living kidney donor informed consent practices vary between US and non-US centers.Nephrol Dial Transplant. 2008 Oct;23(10):3316-24. doi: 10.1093/ndt/gfn295. Epub 2008 Jul 3. Nephrol Dial Transplant. 2008. PMID: 18599559 Free PMC article.
-
A Call for Research on Individuals Who Opt Out of Living Kidney Donation: Challenges and Opportunities.Transplantation. 2016 Dec;100(12):2527-2532. doi: 10.1097/TP.0000000000001408. Transplantation. 2016. PMID: 27495760 Review.
Cited by
-
Pre-emptive live donor kidney transplantation-moving barriers to opportunities: An ethical, legal and psychological aspects of organ transplantation view.World J Transplant. 2021 Apr 18;11(4):88-98. doi: 10.5500/wjt.v11.i4.88. World J Transplant. 2021. PMID: 33954087 Free PMC article. Review.
-
Risk Assessment and Management for Potential Living Kidney Donors: The Role of "Third-Party" Commission.Front Public Health. 2022 Mar 17;10:824048. doi: 10.3389/fpubh.2022.824048. eCollection 2022. Front Public Health. 2022. PMID: 35372186 Free PMC article.
-
Usability assessment of an interactive health technology for kidney living donors: protocol for a prospective cross-sectional survey.BMJ Open. 2022 Jan 3;12(1):e051166. doi: 10.1136/bmjopen-2021-051166. BMJ Open. 2022. PMID: 34980611 Free PMC article.
-
Living Kidney Donor Knowledge of Provided Information and Informed Consent: The PRINCE Study.J Clin Med. 2022 Jan 28;11(3):698. doi: 10.3390/jcm11030698. J Clin Med. 2022. PMID: 35160147 Free PMC article.
References
-
- International Figures on Donation and Transplantation, 2014. http://www.transplant-observatory.org/SiteCollectionDocuments/newsletter...
-
- NTS. NTS Annual report 2014 2015. http://www.transplantatiestichting.nl/sites/defaut/files/product/downloa...
-
- de Klerk M, Kal-van Gestel JA, Haase-Kromwijk BJ et al. . Eight years of outcomes of the Dutch living donor kidney exchange program. Clin Transpl 2011:287–90. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical