A phase I study of high-dose rosuvastatin with standard dose erlotinib in patients with advanced solid malignancies
- PMID: 27036206
- PMCID: PMC4815068
- DOI: 10.1186/s12967-016-0836-6
A phase I study of high-dose rosuvastatin with standard dose erlotinib in patients with advanced solid malignancies
Abstract
Background: Synergistic cytotoxicity with high-dose statins and erlotinib has been demonstrated in preclinical models across a number of tumour types. In this phase I study, we evaluated the safety and potential anti-tumour activity of escalating doses of rosuvastatin in combination with the standard clinical dose of erlotinib in heavily pretreated patients with advanced solid tumours.
Methods: This was a single-center, phase I open-label study to determine the safety and recommended phase two dose (RPTD) of rosuvastatin in combination with 150 mg/day standard dose of erlotinib. Using a 3 + 3 study design and 28-day cycle, escalating doses of rosuvastatin from 1 to 8 mg/kg/day × 2 weeks (cycle 1) and 3 weeks (subsequent cycles) given concurrently with erlotinib were evaluated. In order to expand the experience and to gain additional safety and pharmacokinetic data, two expansions cohorts using concurrent or alternating weekly dosing regimens at the RPTD were also evaluated.
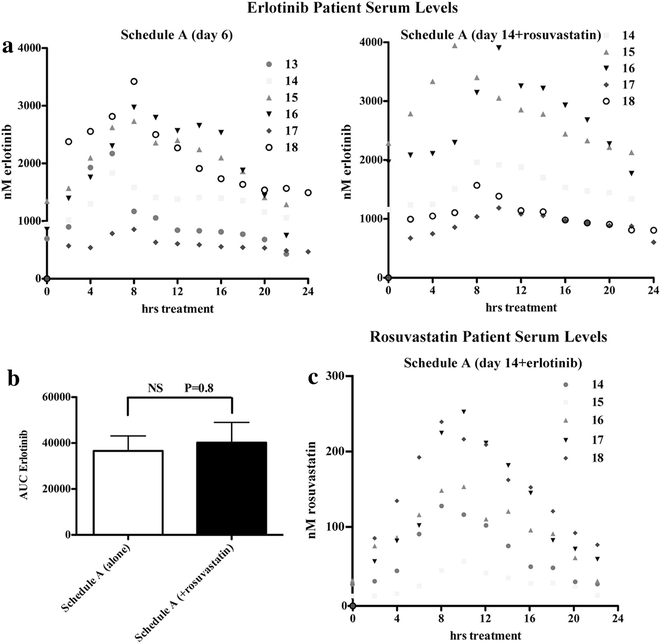
Results: All 24 patients enrolled were evaluable for toxicity, and 22 for response. The dose-limiting toxicity (DLT) of reversible muscle toxicity was seen at the 2 mg/kg/day dose level. Maximal tolerated dose (MTD) was determined to be 1 mg/kg/day. Thirty-three percent of patients developed at least 1 ≥ grade 2 muscle toxicity (rhabdomyolysis: 1/24, myalgia: 7/24) resulting in one study-related death. Durable stable disease for more than 170 days was seen in 25 % of patients that received concurrent treatment and were evaluable for response (n = 16). Plasma erlotinib levels on study were unaffected by the addition of rosuvastatin.
Conclusions: The observed disease stabilization rate of 25 % with combination therapy in this heavily pretreated population is encouraging, however, the high levels of muscle toxicities observed limited this combination strategy.
Keywords: Epidermal growth factor receptor; Erlotinib; Pharmacokinetics; Statins; Therapeutics.
Figures


Similar articles
-
Safety, efficacy, and pharmacokinetics of navitoclax (ABT-263) in combination with erlotinib in patients with advanced solid tumors.Cancer Chemother Pharmacol. 2015 Nov;76(5):1025-32. doi: 10.1007/s00280-015-2883-8. Epub 2015 Sep 29. Cancer Chemother Pharmacol. 2015. PMID: 26420235 Clinical Trial.
-
A Phase I Dose-Escalation Study of the Safety and Pharmacokinetics of Pictilisib in Combination with Erlotinib in Patients with Advanced Solid Tumors.Oncologist. 2017 Dec;22(12):1491-1499. doi: 10.1634/theoncologist.2017-0090. Epub 2017 Aug 10. Oncologist. 2017. PMID: 28798270 Free PMC article. Clinical Trial.
-
Phase I, dose-escalating study of elisidepsin (Irvalec(®)), a plasma membrane-disrupting marine antitumor agent, in combination with erlotinib in patients with advanced malignant solid tumors.Invest New Drugs. 2016 Feb;34(1):75-83. doi: 10.1007/s10637-015-0305-8. Epub 2015 Dec 2. Invest New Drugs. 2016. PMID: 26627080 Clinical Trial.
-
Phase I study of the HER3-targeted antibody patritumab (U3-1287) combined with erlotinib in Japanese patients with non-small cell lung cancer.Lung Cancer. 2015 Jun;88(3):275-81. doi: 10.1016/j.lungcan.2015.03.010. Epub 2015 Mar 19. Lung Cancer. 2015. PMID: 25891541 Clinical Trial.
-
Phase I study of cetuximab, erlotinib, and bevacizumab in patients with advanced solid tumors.Cancer Chemother Pharmacol. 2009 May;63(6):1065-71. doi: 10.1007/s00280-008-0811-x. Epub 2008 Sep 16. Cancer Chemother Pharmacol. 2009. PMID: 18795291 Clinical Trial.
Cited by
-
Statins as anti-tumor agents: A paradigm for repurposed drugs.Cancer Rep (Hoboken). 2024 May;7(5):e2078. doi: 10.1002/cnr2.2078. Cancer Rep (Hoboken). 2024. PMID: 38711272 Free PMC article. Review.
-
Statin as a Potential Chemotherapeutic Agent: Current Updates as a Monotherapy, Combination Therapy, and Treatment for Anti-Cancer Drug Resistance.Pharmaceuticals (Basel). 2021 May 16;14(5):470. doi: 10.3390/ph14050470. Pharmaceuticals (Basel). 2021. PMID: 34065757 Free PMC article. Review.
-
A Narrative Review of Statin-Induced Rhabdomyolysis: Molecular Mechanism, Risk Factors, and Management.Drug Healthc Patient Saf. 2021 Nov 8;13:211-219. doi: 10.2147/DHPS.S333738. eCollection 2021. Drug Healthc Patient Saf. 2021. PMID: 34795533 Free PMC article. Review.
-
Influence of OATPs on Hepatic Disposition of Erlotinib Measured With Positron Emission Tomography.Clin Pharmacol Ther. 2018 Jul;104(1):139-147. doi: 10.1002/cpt.888. Epub 2017 Nov 3. Clin Pharmacol Ther. 2018. PMID: 28940241 Free PMC article.
-
Chemoresistance of TP53 mutant acute myeloid leukemia requires the mevalonate byproduct, geranylgeranyl pyrophosphate, for induction of an adaptive stress response.Leukemia. 2025 Sep;39(9):2087-2098. doi: 10.1038/s41375-025-02668-6. Epub 2025 Jul 9. Leukemia. 2025. PMID: 40634510 Free PMC article.
References
-
- Thomas SM, Coppelli FM, Wells A, Gooding WE, Song J, Kassis J, Drenning SD, Grandis JR. Epidermal growth factor receptor-stimulated activation of phospholipase Cgamma-1 promotes invasion of head and neck squamous cell carcinoma. Cancer Res. 2003;63:5629–5635. - PubMed
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

