X-linked intellectual disability gene CASK regulates postnatal brain growth in a non-cell autonomous manner
- PMID: 27036546
- PMCID: PMC4818453
- DOI: 10.1186/s40478-016-0295-6
X-linked intellectual disability gene CASK regulates postnatal brain growth in a non-cell autonomous manner
Abstract
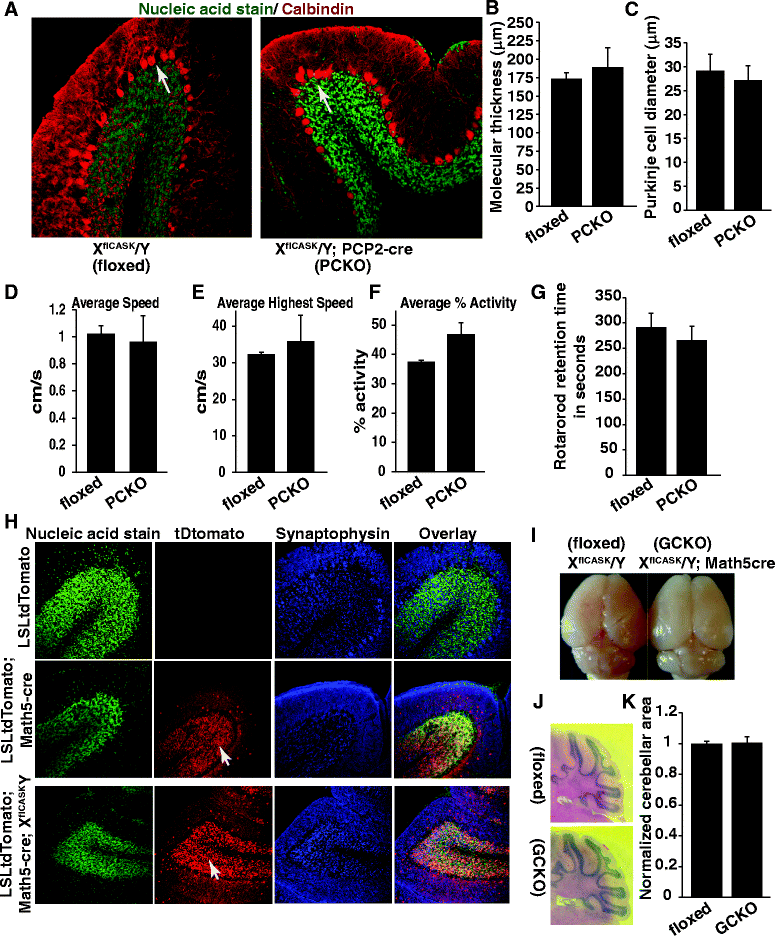
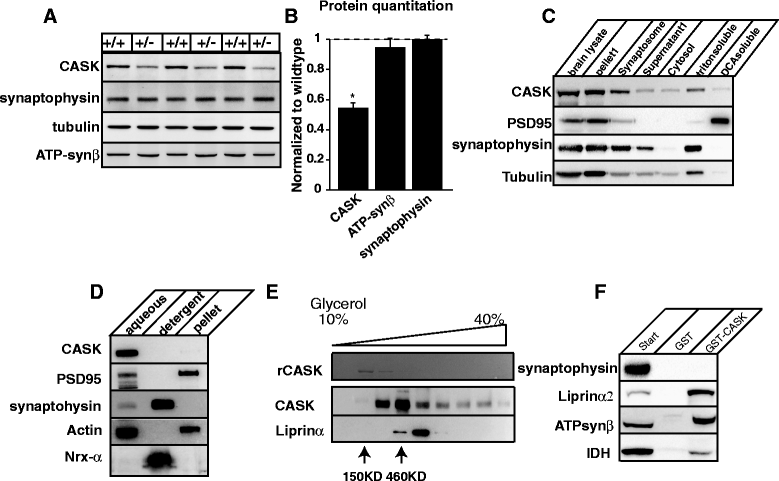
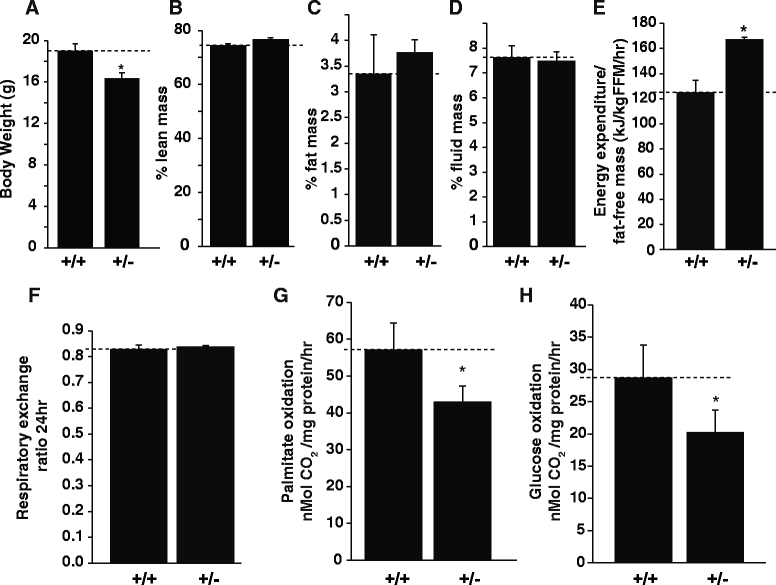
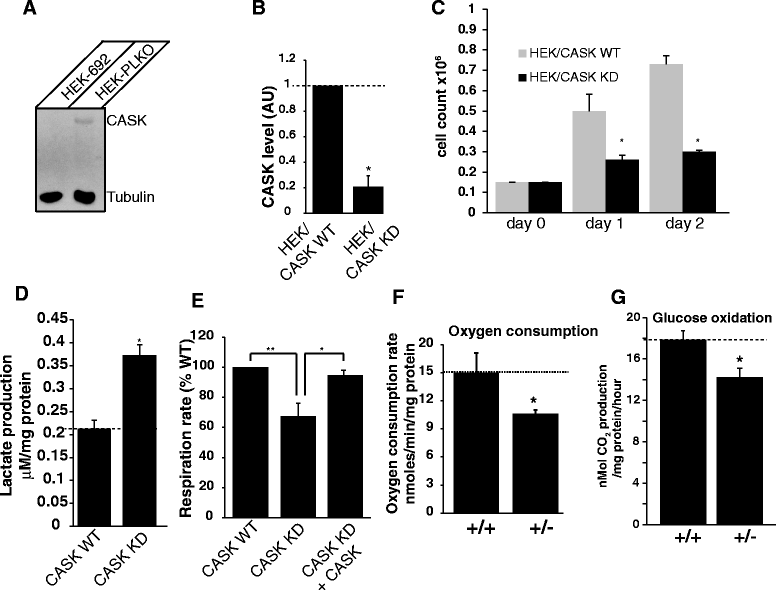
The phenotypic spectrum among girls with heterozygous mutations in the X-linked intellectual disability (XLID) gene CASK (calcium/calmodulin-dependent serine protein kinase) includes postnatal microcephaly, ponto-cerebellar hypoplasia, seizures, optic nerve hypoplasia, growth retardation and hypotonia. Although CASK knockout mice were previously reported to exhibit perinatal lethality and a 3-fold increased apoptotic rate in the brain, CASK deletion was not found to affect neuronal physiology and their electrical properties. The pathogenesis of CASK associated disorders and the potential function of CASK therefore remains unknown. Here, using Cre-LoxP mediated gene excision experiments; we demonstrate that deleting CASK specifically from mouse cerebellar neurons does not alter the cerebellar architecture or function. We demonstrate that the neuron-specific deletion of CASK in mice does not cause perinatal lethality but induces severe recurrent epileptic seizures and growth retardation before the onset of adulthood. Furthermore, we demonstrate that although neuron-specific haploinsufficiency of CASK is inconsequential, the CASK mutation associated human phenotypes are replicated with high fidelity in CASK heterozygous knockout female mice (CASK ((+/-))). These data suggest that CASK-related phenotypes are not purely neuronal in origin. Surprisingly, the observed microcephaly in CASK ((+/-)) animals is not associated with a specific loss of CASK null brain cells indicating that CASK regulates postnatal brain growth in a non-cell autonomous manner. Using biochemical assay, we also demonstrate that CASK can interact with metabolic proteins. CASK knockdown in human cell lines cause reduced cellular respiration and CASK ((+/-)) mice display abnormalities in muscle and brain oxidative metabolism, suggesting a novel function of CASK in metabolism. Our data implies that some phenotypic components of CASK heterozygous deletion mutation associated disorders represent systemic manifestation of metabolic stress and therefore amenable to therapeutic intervention.
Keywords: CASK; Cerebellar hypoplasia; MAGUK; Metabolism; Non-cell autonomous; X-linked intellectual disability.
Figures



References
-
- Birkebaek NH, Patel L, Wright NB, Grigg JR, Sinha S, Hall CM, Price DA, Lloyd IC, Clayton PE. Endocrine status in patients with optic nerve hypoplasia: relationship to midline central nervous system abnormalities and appearance of the hypothalamic-pituitary axis on magnetic resonance imaging. J Clin Endocrinol Metab. 2003;88:5281–5286. doi: 10.1210/jc.2003-030527. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

