Intradermal injection of a Tat Oyi-based therapeutic HIV vaccine reduces of 1.5 log copies/mL the HIV RNA rebound median and no HIV DNA rebound following cART interruption in a phase I/II randomized controlled clinical trial
- PMID: 27036656
- PMCID: PMC4818470
- DOI: 10.1186/s12977-016-0251-3
Intradermal injection of a Tat Oyi-based therapeutic HIV vaccine reduces of 1.5 log copies/mL the HIV RNA rebound median and no HIV DNA rebound following cART interruption in a phase I/II randomized controlled clinical trial
Erratum in
-
Erratum to: Intradermal injection of a Tat Oyi-based therapeutic HIV vaccine reduces of 1.5 log copies/mL the HIV RNA rebound median and no HIV DNA rebound following cART interruption in a phase I/II randomized controlled clinical trial.Retrovirology. 2016 May 9;13(1):35. doi: 10.1186/s12977-016-0264-y. Retrovirology. 2016. PMID: 27160192 Free PMC article. No abstract available.
Abstract
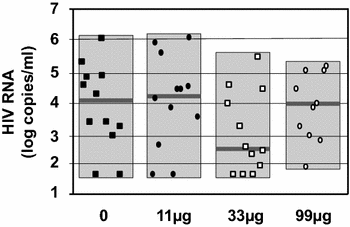
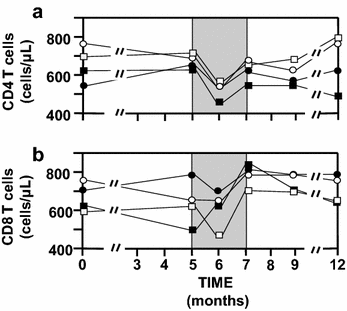
Background: A Tat Oyi vaccine preparation was administered with informed consent to 48 long-term HIV-1 infected volunteers whose viral loads had been suppressed by antiretroviral therapy (cART). These volunteers were randomized in double-blind method into four groups (n = 12) that were injected intradermally with 0, 11, 33, or 99 µg of synthetic Tat Oyi proteins in buffer without adjuvant at times designated by month 0 (M0), M1 and M2, respectively. The volunteers then underwent a structured treatment interruption between M5 and M7.
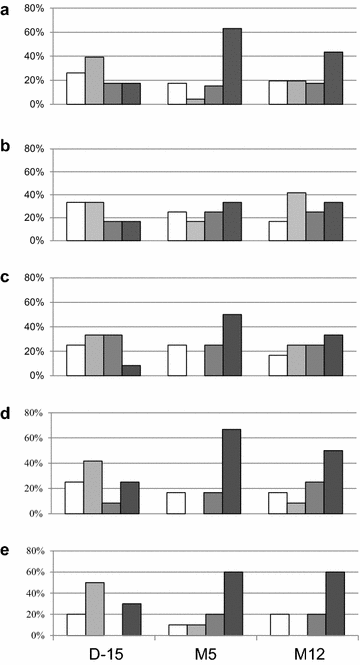
Results: The primary outcomes of this phase I/IIa clinical trial were the safety and lowering the extent of HIV RNA rebound after cART interruption. Only one undesirable event possibly due to vaccination was observed. The 33 µg dose was most effective at lowering the extent of HIV RNA and DNA rebound (Mann and Whitney test, p = 0.07 and p = 0.001). Immune responses against Tat were increased at M5 and this correlated with a low HIV RNA rebound at M6 (p = 0.01).
Conclusion: This study suggests in vivo that extracellular Tat activates and protects HIV infected cells. The Tat Oyi vaccine in association with cART may provide an efficient means of controlling the HIV-infected cell reservoir.
Keywords: ART interruption; Clinical trial; HIV; Tat; Vaccine.
Figures



References
-
- Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vézinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. - DOI - PubMed
-
- Gallo RC, Salahuddin SZ, Popovic M, Shearer GM, Kaplan M, Haynes BF, Palker TJ, Redfield R, Oleske J, Safai B, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. - DOI - PubMed
-
- Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, Lai J, McHugh HL, Hao H, Zhang H, Margolick JB, Gurer C, Murphy AJ, Valenzuela DM, Yancopoulos GD, Deeks SG, Strowig T, Kumar P, Siliciano JD, Salzberg SL, Flavell RA, Shan L, Siliciano RF. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517:381–385. doi: 10.1038/nature14053. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

