Mucin-1 Increases Renal TRPV5 Activity In Vitro, and Urinary Level Associates with Calcium Nephrolithiasis in Patients
- PMID: 27036738
- PMCID: PMC5084893
- DOI: 10.1681/ASN.2015101100
Mucin-1 Increases Renal TRPV5 Activity In Vitro, and Urinary Level Associates with Calcium Nephrolithiasis in Patients
Abstract
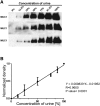
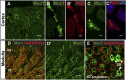
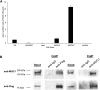
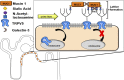
Hypercalciuria is a major risk factor for nephrolithiasis. We previously reported that Uromodulin (UMOD) protects against nephrolithiasis by upregulating the renal calcium channel TRPV5. This channel is crucial for calcium reabsorption in the distal convoluted tubule (DCT). Recently, mutations in the gene encoding Mucin-1 (MUC1) were found to cause autosomal dominant tubulointerstitial kidney disease, the same disease caused by UMOD mutations. Because of the similarities between UMOD and MUC1 regarding associated disease phenotype, protein structure, and function as a cellular barrier, we examined whether urinary MUC1 also enhances TRPV5 channel activity and protects against nephrolithiasis. We established a semiquantitative assay for detecting MUC1 in human urine and found that, compared with controls (n=12), patients (n=12) with hypercalciuric nephrolithiasis had significantly decreased levels of urinary MUC1. Immunofluorescence showed MUC1 in the thick ascending limb, DCT, and collecting duct. Applying whole-cell patch-clamp recording of HEK cells, we found that wild-type but not disease mutant MUC1 increased TRPV5 activity by impairing dynamin-2- and caveolin-1-mediated endocytosis of TRPV5. Coimmunoprecipitation confirmed a physical interaction between TRPV5 and MUC1. However, MUC1 did not increase the activity of N-glycan-deficient TRPV5. MUC1 is characterized by variable number tandem repeats (VNTRs) that bind the lectin galectin-3; galectin-3 siRNA but not galectin-1 siRNA prevented MUC1-induced upregulation of TRPV5 activity. Additionally, MUC1 lacking VNTRs did not increase TRPV5 activity. Our results suggest that MUC1 forms a lattice with the N-glycan of TRPV5 via galectin-3, which impairs TRPV5 endocytosis and increases urinary calcium reabsorption.
Keywords: calcium; electrophysiology; endocytosis; hypercalciuria; ion channel; kidney stones.
Copyright © 2016 by the American Society of Nephrology.
Figures





Comment in
-
Re: Mucin-1 Increases Renal TRPV5 Activity In Vitro, and Urinary Level Associates with Calcium Nephrolithiasis in Patients.J Urol. 2016 Nov;196(5):1471-1472. doi: 10.1016/j.juro.2016.08.020. Epub 2016 Aug 23. J Urol. 2016. PMID: 27751461 No abstract available.
-
Re: Mucin-1 Increases Renal TRPV5 Activity In Vitro, and Urinary Level Associates with Calcium Nephrolithiasis in Patients.J Urol. 2017 Jul;198(1):138-139. doi: 10.1016/j.juro.2017.04.057. Epub 2017 Apr 12. J Urol. 2017. PMID: 28618678 No abstract available.
References
-
- Kumar V, Lieske JC: Protein regulation of intrarenal crystallization. Curr Opin Nephrol Hypertens 15: 374–380, 2006 - PubMed
-
- Frick KK, Bushinsky DA: Molecular mechanisms of primary hypercalciuria. J Am Soc Nephrol 14: 1082–1095, 2003 - PubMed
-
- Costanzo LS, Windhager EE, Ellison DH: Calcium and sodium transport by the distal convoluted tubule of the rat. 1978. J Am Soc Nephrol 11: 1562–1580, 2000 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

