Upregulation of calpain activity precedes tau phosphorylation and loss of synaptic proteins in Alzheimer's disease brain
- PMID: 27036949
- PMCID: PMC4818436
- DOI: 10.1186/s40478-016-0299-2
Upregulation of calpain activity precedes tau phosphorylation and loss of synaptic proteins in Alzheimer's disease brain
Abstract
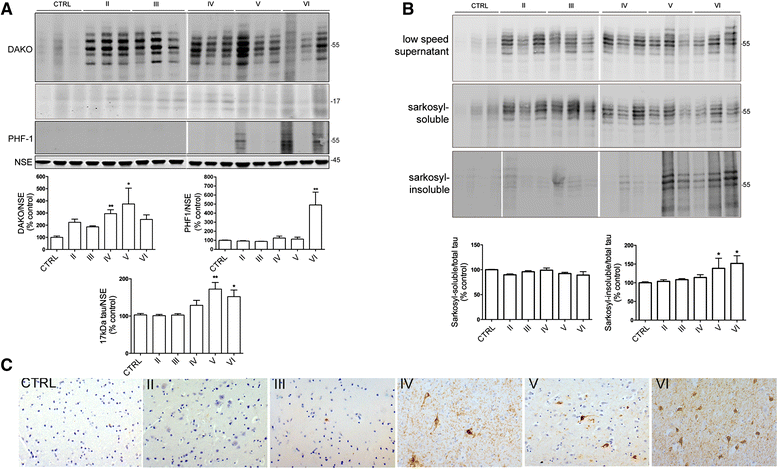
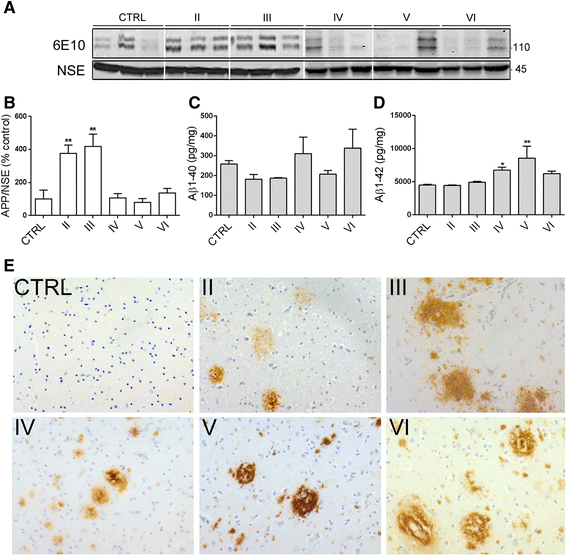
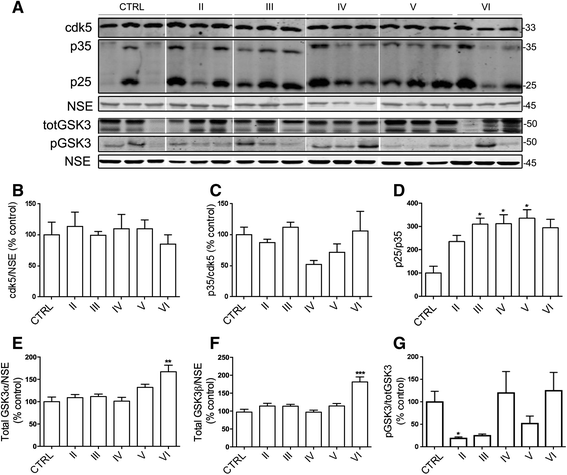
Alterations in calcium homeostasis are widely reported to contribute to synaptic degeneration and neuronal loss in Alzheimer's disease. Elevated cytosolic calcium concentrations lead to activation of the calcium-sensitive cysteine protease, calpain, which has a number of substrates known to be abnormally regulated in disease. Analysis of human brain has shown that calpain activity is elevated in AD compared to controls, and that calpain-mediated proteolysis regulates the activity of important disease-associated proteins including the tau kinases cyclin-dependent kinase 5 and glycogen kinase synthase-3. Here, we sought to investigate the likely temporal association between these changes during the development of sporadic AD using Braak staged post-mortem brain. Quantification of protein amounts in these tissues showed increased activity of calpain-1 from Braak stage III onwards in comparison to controls, extending previous findings that calpain-1 is upregulated at end-stage disease, and suggesting that activation of calcium-sensitive signalling pathways are sustained from early stages of disease development. Increases in calpain-1 activity were associated with elevated activity of the endogenous calpain inhibitor, calpastatin, itself a known calpain substrate. Activation of the tau kinases, glycogen-kinase synthase-3 and cyclin-dependent kinase 5 were also found to occur in Braak stage II-III brain, and these preceded global elevations in tau phosphorylation and the loss of post-synaptic markers. In addition, we identified transient increases in total amyloid precursor protein and pre-synaptic markers in Braak stage II-III brain, that were lost by end stage Alzheimer's disease, that may be indicative of endogenous compensatory responses to the initial stages of neurodegeneration. These findings provide insight into the molecular events that underpin the progression of Alzheimer's disease, and further highlight the rationale for investigating novel treatment strategies that are based on preventing abnormal calcium homeostasis or blocking increases in the activity of calpain or important calpain substrates.
Keywords: Alzheimer’s disease; Braak stage; Calpain; GSK-3; Postmortem brain; Synapse; Tau.
Figures



References
-
- Ando K, Brion JP, Stygelbout V, Suain V, Authelet M, Dedecker R, Chanut A, Lacor P, Lavaur J, Sazdovitch V, Rogaeva E, Potier MC, Duyckaerts C. Clathrin adaptor CALM/PICALM is associated with neurofibrillary tangles and is cleaved in Alzheimer’s brains. Acta Neuropathol. 2013;125:861–878. doi: 10.1007/s00401-013-1111-z. - DOI - PubMed
-
- Bayés À, Collins MO, Galtrey CM, Simonnet C, Roy M, Croning MD, Gou G, van de Lagemaat LN, Milward D, Whittle IR, Smith C, Choudhary JS, Grant SG. Human post-mortem synapse proteome integrity screening for proteomic studies of postsynaptic complexes. Mol Brain. 2014;7:88. doi: 10.1186/s13041-014-0088-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

