Retention in care during the first 3 years of antiretroviral therapy for women in Malawi's option B+ programme: an observational cohort study
- PMID: 27036993
- PMCID: PMC4904064
- DOI: 10.1016/S2352-3018(16)00008-4
Retention in care during the first 3 years of antiretroviral therapy for women in Malawi's option B+ programme: an observational cohort study
Abstract
Background: Studies of Malawi's option B+ programme for HIV-positive pregnant and breastfeeding women have reported high loss to follow-up during pregnancy and at the start of antiretroviral therapy (ART), but few data exist about retention during breastfeeding and after weaning. We examined loss to follow-up and retention in care in patients in the option B+ programme during their first 3 years on ART.
Methods: We analysed two data sources: aggregated facility-level data about patients in option B+ who started ART between Oct 1, 2011, and June 30, 2012, at 546 health facilities; and patient-level data from 20 large facilities with electronic medical record system for HIV-positive women who started ART between Sept 1, 2011, and Dec 31, 2013, under option B+ or because they had WHO clinical stages 3 or 4 disease or had CD4 counts of less than 350 cells per μL. We used facility-level data to calculate representative estimates of retention and loss to follow-up. We used patient-level data to study temporal trends in retention, timing of loss to follow-up, and predictors of no follow-up and loss to follow-up. We defined patients who were more than 60 days late for their first follow-up visit as having no follow-up and patients who were more than 60 days late for a subsequent visit as being lost to follow-up. We calculated proportions and cumulative probabilities of patients who had died, stopped ART, had no follow-up, were lost to follow-up, or were retained alive on ART for 36 months. We calculated odds ratios and hazard ratios to examine predictors of no follow-up and loss to follow-up.
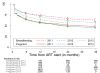
Findings: Analysis of facility-level data about patients in option B+ who had not transferred to a different facility showed retention in care to be 76·8% (20 475 of 26,658 patients) after 12 months, 70·8% (18,306 of 25,849 patients) after 24 months, and 69·7% (17,787 of 25,535 patients) after 36 months. Patient-level data included 29,145 patients. 14,630 (50·2%) began treatment under option B+. Patients in option B+ had a higher risk of having no follow-up and, for the first 2 years of ART, higher risk of loss to follow-up than did patients who started ART because they had CD4 counts less than 350 cells per μL or WHO clinical stage 3 or 4 disease. Risk of loss to follow-up during the third year was low and similar for patients retained for 2 years. Retention rates did not change as the option B+ programme matured.
Interpretation: Our data suggest that pregnant and breastfeeding women who start ART immediately after they are diagnosed with HIV can be retained on ART through the option B+ programme, even after many have stopped breastfeeding. Interventions might be needed to improve retention in the first year on ART in option B+.
Funding: Bill & Melinda Gates Foundation, Partnerships for Enhanced Engagement in Research Health, and National Institute of Allergy and Infectious Diseases.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
We declare no competing interests.
Figures

Comment in
-
Good news for retention of women on option B+ in Malawi.Lancet HIV. 2016 Apr;3(4):e151-2. doi: 10.1016/S2352-3018(16)00035-7. Epub 2016 Mar 9. Lancet HIV. 2016. PMID: 27036987 No abstract available.
-
Profile: Swiss School of Public Health, Zurich, Switzerland.Lancet. 2017 Jan 14;389(10065):144. doi: 10.1016/S0140-6736(17)30080-6. Epub 2017 Jan 13. Lancet. 2017. PMID: 28102131 No abstract available.
References
-
- Schouten EJ, Jahn A, Midiani D, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011;378:282–4. - PubMed
-
- Ministry of Health Malawi. Clinical Management of HIV In Children and Adults. 2014 http://cms.medcol.mw/cms_uploaded_resources/18381_16.pdf.
-
- World Health Organization. Global update on HIV treatment 2013: results, impact and opportunities. 2013 http://apps.who.int/iris/bitstream/10665/85326/1/9789241505734_eng.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

