Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders
- PMID: 27037223
- PMCID: PMC4819215
- DOI: 10.1124/pr.115.010652
Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders
Abstract
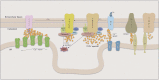
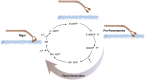
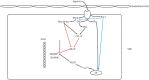
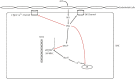
The smooth muscle cell directly drives the contraction of the vascular wall and hence regulates the size of the blood vessel lumen. We review here the current understanding of the molecular mechanisms by which agonists, therapeutics, and diseases regulate contractility of the vascular smooth muscle cell and we place this within the context of whole body function. We also discuss the implications for personalized medicine and highlight specific potential target molecules that may provide opportunities for the future development of new therapeutics to regulate vascular function.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures





References
-
- Abassi ZA, Gurbanov K, Mulroney SE, Potlog C, Opgenorth TJ, Hoffman A, Haramati A, Winaver J. (1997) Impaired nitric oxide-mediated renal vasodilation in rats with experimental heart failure: role of angiotensin II. Circulation 96:3655–3664. - PubMed
-
- Alahyan M, Webb MR, Marston SB, El-Mezgueldi M. (2006) The mechanism of smooth muscle caldesmon-tropomyosin inhibition of the elementary steps of the actomyosin ATPase. J Biol Chem 281:19433–19448. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

