Interplay between Fanconi anemia and homologous recombination pathways in genome integrity
- PMID: 27037238
- PMCID: PMC4865030
- DOI: 10.15252/embj.201693860
Interplay between Fanconi anemia and homologous recombination pathways in genome integrity
Abstract
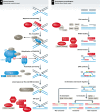
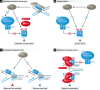
The Fanconi anemia (FA) pathway plays a central role in the repair of DNA interstrand crosslinks (ICLs) and regulates cellular responses to replication stress. Homologous recombination (HR), the error-free pathway for double-strand break (DSB) repair, is required during physiological cell cycle progression for the repair of replication-associated DNA damage and protection of stalled replication forks. Substantial crosstalk between the two pathways has recently been unravelled, in that key HR proteins such as the RAD51 recombinase and the tumour suppressors BRCA1 and BRCA2 also play important roles in ICL repair. Consistent with this, rare patient mutations in these HR genes cause FA pathologies and have been assigned FA complementation groups. Here, we focus on the clinical and mechanistic implications of the connection between these two cancer susceptibility syndromes and on how these two molecular pathways of DNA replication and repair interact functionally to prevent genomic instability.
Keywords: DNA damage response; DNA repair; Fanconi anemia; genome stability; homologous recombination; replication stress.
© 2016 The Authors. Published under the terms of the CC BY 4.0 license.
Figures
References
-
- Alter BP (1996) Fanconi's anemia and malignancies. Am J Hematol 53: 99–110 - PubMed
-
- Alter BP (2006) The association between FANCD1/BRCA2 mutations and leukaemia. Br J Haematol 133: 446–448 - PubMed
-
- Ameziane N, May P, Haitjema A, van de Vrugt HJ, van Rossum‐Fikkert SE, Ristic D, Williams GJ, Balk J, Rockx D, Li H, Rooimans MA, Oostra AB, Velleuer E, Dietrich R, Bleijerveld OB, Maarten Altelaar AF, Meijers‐Heijboer H, Joenje H, Glusman G, Roach J et al (2015) A novel Fanconi anaemia subtype associated with a dominant‐negative mutation in RAD51. Nat Commun 6: 8829 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

