Molecular Pathways: Breaking the Epithelial Cancer Barrier for Chimeric Antigen Receptor and T-cell Receptor Gene Therapy
- PMID: 27037253
- PMCID: PMC4877620
- DOI: 10.1158/1078-0432.CCR-15-1294
Molecular Pathways: Breaking the Epithelial Cancer Barrier for Chimeric Antigen Receptor and T-cell Receptor Gene Therapy
Abstract
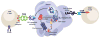
Adoptive transfer of T cells genetically engineered to express a tumor-targeting chimeric antigen receptor (CAR) or T-cell receptor (TCR) can mediate cancer regression in some patients. CARs are synthetic single-chain proteins that use antibody domains to target cell surface antigens. TCRs are natural heterodimeric proteins that can target intracellular antigens through recognition of peptides bound to human leukocyte antigens. CARs have shown promise in B-cell malignancies and TCRs in melanoma, but neither approach has achieved clear success in an epithelial cancer. Treatment of epithelial cancers may be particularly challenging because of a paucity of target antigens expressed by carcinomas and not by important healthy tissues. In addition, epithelial cancers may be protected by inhibitory ligands and soluble factors in the tumor microenvironment. One strategy to overcome these negative regulators is to modulate expression of T-cell genes to enhance intrinsic T-cell function. Programmable nucleases, which can suppress inhibitory genes, and inducible gene expression systems, which can enhance stimulatory genes, are entering clinical testing. Other work is delineating whether control of genes for immune checkpoint receptors (e.g.,PDCD1, CTLA4) and cytokine and TCR signaling regulators (e.g.,CBLB, CISH, IL12, IL15) can increase the antitumor activity of therapeutic T cells.
©2016 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
Figures

References
-
- Kochenderfer JN, Dudley ME, Kassim SH, Somerville RPT, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–9. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

