Signals and Receptors
- PMID: 27037414
- PMCID: PMC4817805
- DOI: 10.1101/cshperspect.a005900
Signals and Receptors
Abstract
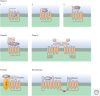
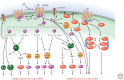
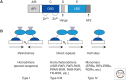
Communication between cells in a multicellular organism occurs by the production of ligands (proteins, peptides, fatty acids, steroids, gases, and other low-molecular-weight compounds) that are either secreted by cells or presented on their surface, and act on receptors on, or in, other target cells. Such signals control cell growth, migration, survival, and differentiation. Signaling receptors can be single-span plasma membrane receptors associated with tyrosine or serine/threonine kinase activities, proteins with seven transmembrane domains, or intracellular receptors. Ligand-activated receptors convey signals into the cell by activating signaling pathways that ultimately affect cytosolic machineries or nuclear transcriptional programs or by directly translocating to the nucleus to regulate transcription.
Copyright © 2016 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



References
-
- Aggarwal BB. 2003. Signalling pathways of the TNF superfamily: A double-edged sword. Nat Rev Immunol 3: 745–756. - PubMed
-
- Amit I, Citri A, Shay T, Lu Y, Katz M, Zhang F, Tarcic G, Siwak D, Lahad J, Jacob-Hirsch J, et al. 2007. A module of negative feedback regulators defines growth factor signaling. Nat Genet 39: 503–512. - PubMed
-
- Anastasiadis PZ, Reynolds AB. 2001. Regulation of Rho GTPases by p120-catenin. Curr Opin Cell Biol 13: 604. - PubMed
-
- Ancot F, Foveau B, Lefebvre J, Leroy C, Tulasne D. 2009. Proteolytic cleavages give receptor tyrosine kinases the gift of ubiquity. Oncogene 28: 2185–2195. - PubMed
-
- Andl CD, Mizushima T, Nakagawa H, Oyama K, Harada H, Chruma K, Herlyn M, Rustgi AK. 2003. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J Biol Chem 278: 1824–1830. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
