What is new in nanoparticle-based photoacoustic imaging?
- PMID: 27038222
- PMCID: PMC5045757
- DOI: 10.1002/wnan.1404
What is new in nanoparticle-based photoacoustic imaging?
Abstract
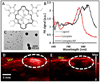
Photoacoustic imaging combines the high temporal and spatial resolution of ultrasound with the good contrast and spectral tuning of optical imaging. Contrast agents are used in photoacoustic imaging to further increase the contrast and specificity of imaging or to image a specific molecular process, e.g., cell-surface proteins or small molecule biomarkers. Nanoparticle-based contrast agents are important tools in photoacoustic imaging because they offer intense and stable signal and can be targeted to specific molecular processes. In this review, we describe some of the most interesting and recent advances in nanoparticle-based photoacoustic imaging including inorganic nanoparticles, organic/polymeric nanoparticles, nanoparticle coatings, multimodality imaging, as well as emerging topics in the field. WIREs Nanomed Nanobiotechnol 2017, 9:e1404. doi: 10.1002/wnan.1404 For further resources related to this article, please visit the WIREs website.
© 2016 Wiley Periodicals, Inc.
Figures





References
-
- James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiological Rev. 2012;92:897–965. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

