A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study
- PMID: 27038608
- PMCID: PMC4818886
- DOI: 10.1186/s13075-016-0981-6
A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study
Abstract
Background: CT-P13 (Remsima®, Inflectra®) is a biosimilar of the infliximab reference product (RP; Remicade®). The aim of this study was to compare the 54-week efficacy, immunogenicity, safety, pharmacokinetics (PK) and pharmacodynamics (PD) of CT-P13 and RP in patients with active rheumatoid arthritis (RA).
Methods: In this multinational phase III double-blind study, patients with active RA and an inadequate response to methotrexate (MTX) were randomized (1:1) to receive CT-P13 (3 mg/kg) or RP (3 mg/kg) at weeks 0, 2, 6 and then every 8 weeks to week 54 in combination with MTX (12.5-25 mg/week). Efficacy endpoints included American College of Rheumatology (ACR)20, ACR50 and ACR70 response rates, Disease Activity Score in 28 joints (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), European League Against Rheumatism (EULAR) response rates, patient-reported outcomes and joint damage progression. Immunogenicity, safety and PK/PD outcomes were also assessed.
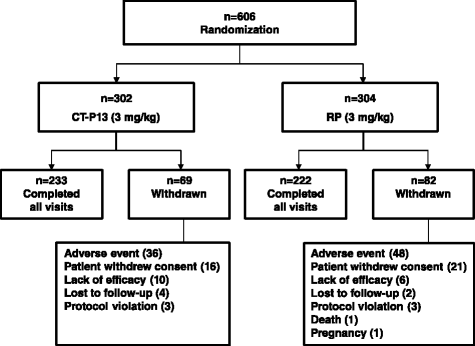
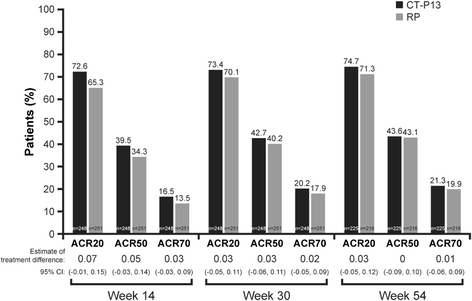
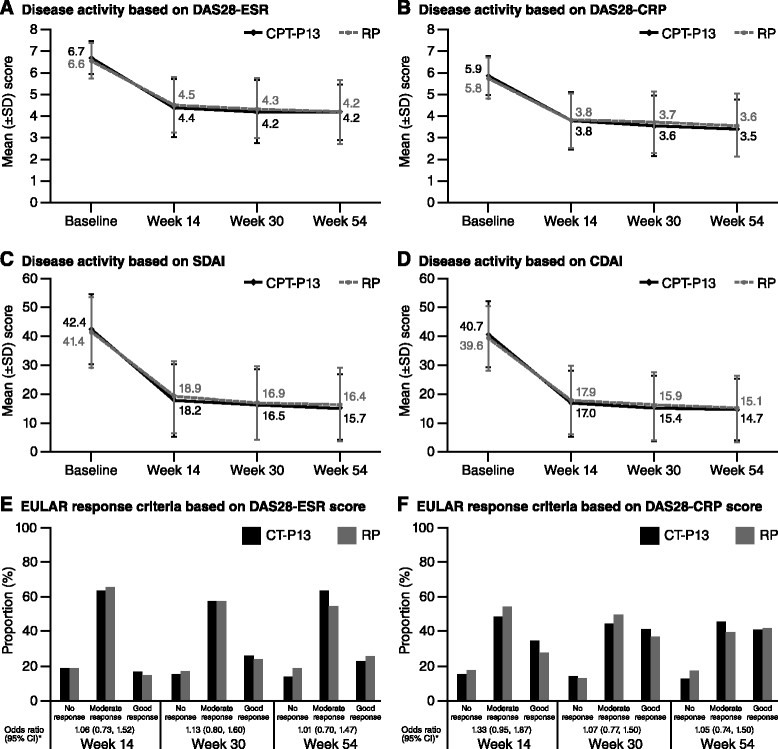
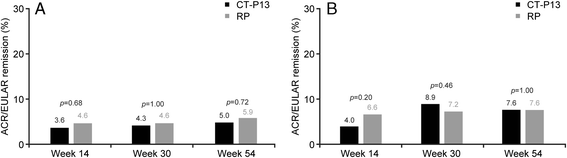
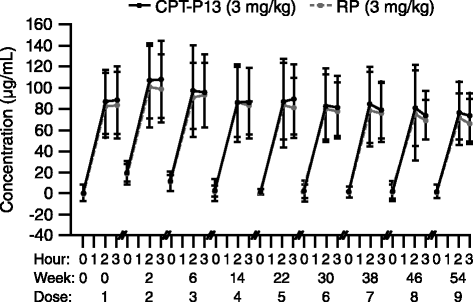
Results: Of 606 randomized patients, 455 (CT-P13 233, RP 222) were treated up to week 54. At week 54, ACR20 response rate was highly similar between groups (CT-P13 74.7 %, RP 71.3 %). ACR50 and ACR70 response rates were also comparable between groups (CT-P13 43.6 % and 21.3 %, respectively; RP 43.1 % and 19.9 %, respectively). DAS28, SDAI and CDAI decreased from baseline to week 54 to a similar extent with CT-P13 and RP. Radiographic progression measured by Sharp scores as modified by van der Heijde was also comparable. With both treatments, patient assessments of pain, disease activity and physical ability, as well as mean scores on the Medical Outcomes Study Short Form Health Survey (SF-36), improved markedly at week 14 and remained stable thereafter up to week 54. The proportion of patients positive for antidrug antibodies at week 54 was similar between the two groups: 41.1 % and 36.0 % with CT-P13 and RP, respectively. CT-P13 was well tolerated and had a similar safety profile to RP. PK/PD results were also comparable between CT-P13 and RP.
Conclusions: CT-P13 and RP were comparable in terms of efficacy (including radiographic progression), immunogenicity and PK/PD up to week 54. The safety profile of CT-P13 was also similar to that of RP.
Trial registration: ClinicalTrials.gov identifier: NCT01217086 . Registered 4 Oct 2010.
Keywords: ACR20; Biosimilar; CT-P13; Efficacy; Immunogenicity; Infliximab; Pharmacokinetics; Rheumatoid arthritis; Safety; Sharp score.
Figures
References
-
- Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor α monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet. 1999;354:1932–9. doi: 10.1016/S0140-6736(99)05246-0. - DOI - PubMed
-
- Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor α monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–63. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

